Transgenic vector system capable of promoting cell transplantation and gene expression and applications of transgenic vector system
A transgenic carrier and gene expression technology, applied in the direction of vectors, nucleic acid carriers, and the use of vectors to introduce foreign genetic material, etc., can solve problems such as hindering applications, uncommon characteristics of target genes, and restricting use, so as to achieve great application value and avoid drug toxicity the effect of the influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. In vivo screening system using BCL2 mutant S70A as screening gene
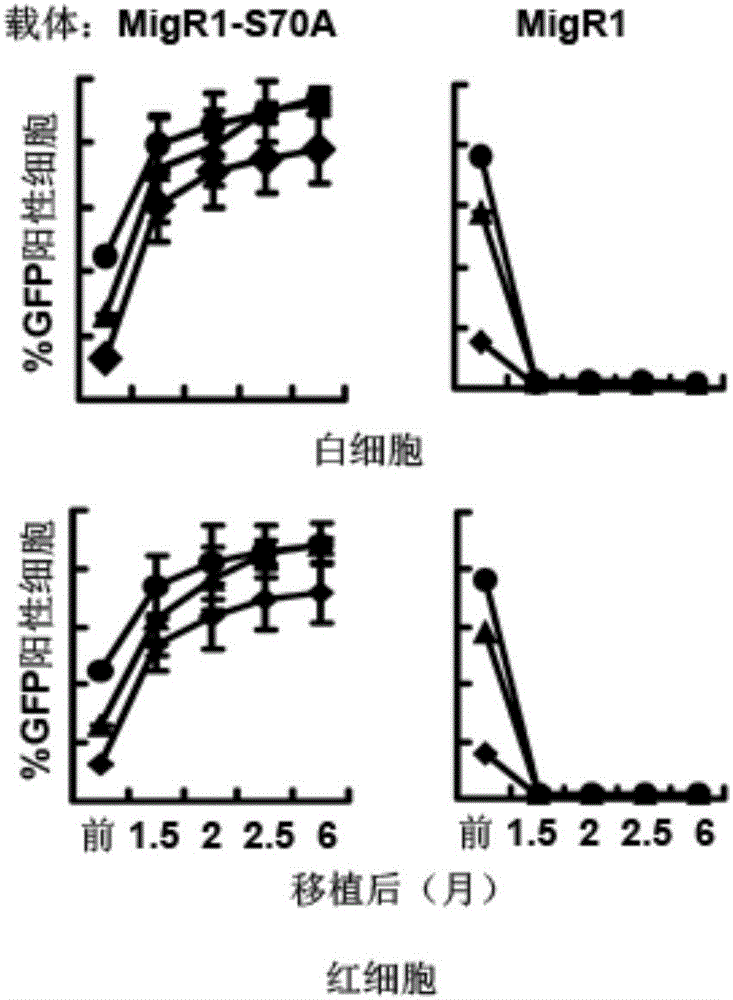
[0039] In Example 1, the BCL2 mutant S70A was used as the screening gene, and the retroviral vector MIGR1 was used as the vector. As mentioned above, S70A retains part of the anti-apoptotic ability of BCL2, but removes its tumor-promoting ability, so it is particularly suitable as a natural screening gene in vivo.
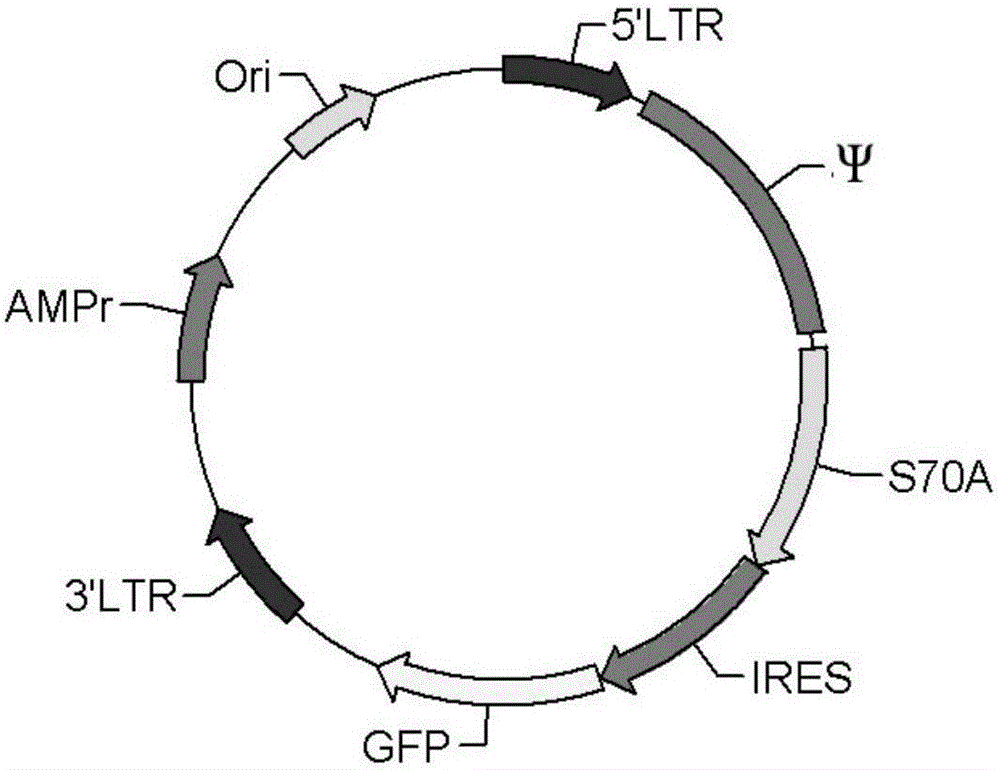
[0040] (1) Insert S70A before the IRES of MIGR1, which is the MIGR1-S70A plasmid, such as figure 1 shown.
[0041] (2) The retroviral supernatant was prepared with MIGR1-S70A plasmid and packaging cell BOSC23. Specifically, BOSC23 cells were cultured in 6-well cell culture plates in complete medium (Dulbecco's Modified Eagle's Medium, DMEM) complete medium (adding 10% fetal bovine serum, 100 units / ml penicillin, 100 μg / ml streptomycin, 2mM L-glutamine, etc.) (37°C, 5% CO 2 ), when the cells are 90% confluent, replace the old medium with fresh DMEM medium containing 5% FBS and ...
Embodiment 2
[0048] Example 2. In vivo screening system based on induction system and lentiviral vector using Bcl-2 mutant S70A as screening gene
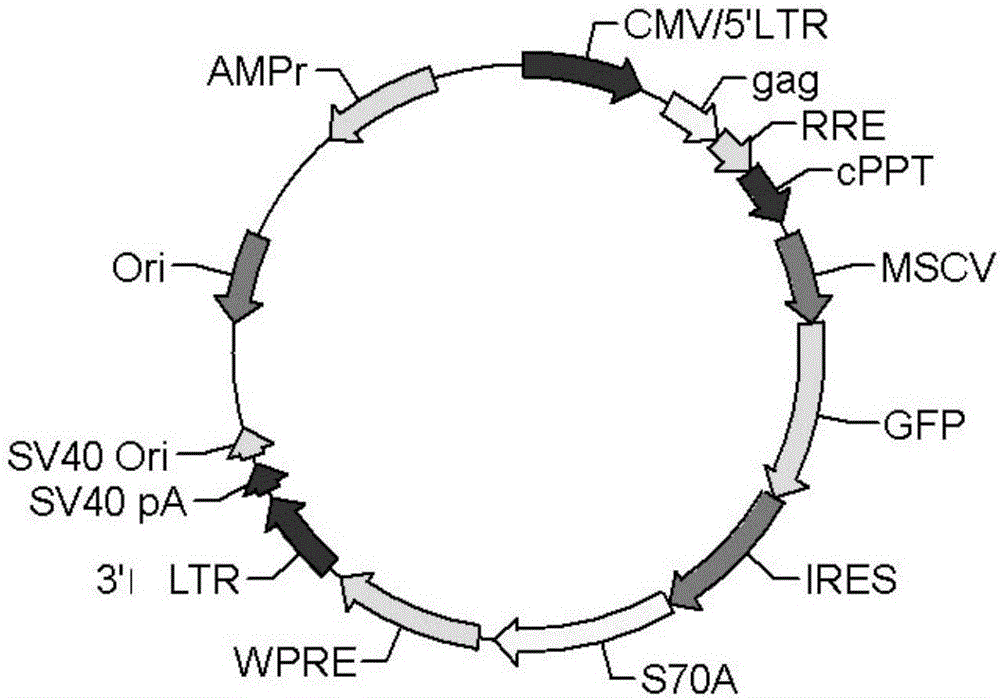
[0049] Lentiviruses have safer properties than retroviruses. Therefore repeat the research of embodiment 1 with self-inactivating feline immunodeficiency virus ( image 3 , the empty viral vector was from SBI Company). The packaging cells used for virus production are 293T cells. In addition to the lentiviral vector, two other packaging plasmids (pFIV-34N, pVSV-G) should be transferred into the 293T cells. The lentivirus packaging method, bone marrow cell acquisition, virus infection and cell transplantation are the same as in Example 1.
[0050] Six months after transplantation, it was found that lentivirus-based S70A could increase the proportion of GFP-positive cells, but unlike retrovirus S70A, lentivirus S70A could only increase the proportion of GFP-positive cells to a low level, about 30%. By comparing the fluorescence intensity value...
Embodiment 3
[0051] Example 3. Non-myeloablative pre-transplant conditioning by 5-FU treatment
[0052] In clinical practice, transplant recipient patients often cannot use lethal doses of radiation to irradiate myeloablation, so it is very critical whether non-myeloablative pre-transplantation treatment with drugs is feasible. Before transplantation, the recipient mice were injected with 150 mg / kg body weight of pentafluorouracil (5-FU). Six months after transplantation, it was found that this pretreatment was sufficient to successfully transplant bone marrow cells infected by retrovirus MIGR1-S70A ( Figure 4 ), so this method is very suitable for clinical application.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com