Liver stem cell injection and preparation method thereof
A technology of hepatic stem cells and injections, applied in the field of biomedicine, can solve problems such as the establishment of safe and reliable human hepatic stem cell lines, and achieve better therapeutic effects, prolong the number of passages, and shorten the growth cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
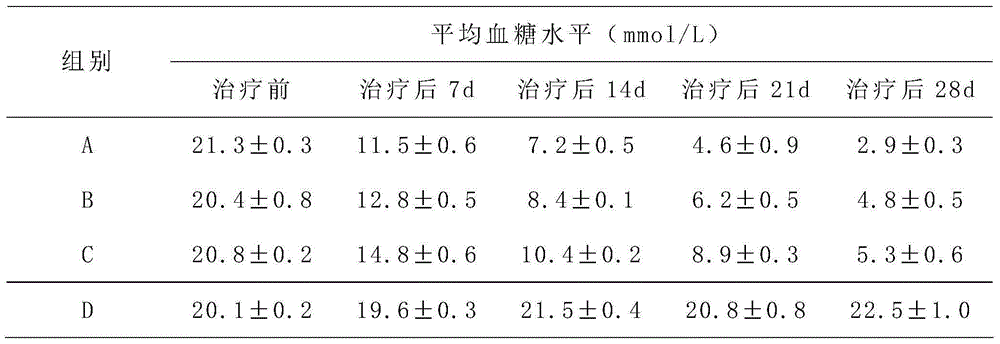
[0033] A hepatic stem cell injection for treating diabetes, each ml injection mixture contains 1×10 7 hepatic stem cells and 1×10 3 umbilical cord mesenchymal stem cells; the injection mixture consists of the following mass percentage components: 2% polyethylene glycol, 2% arginine, 3% lysine, 2% methionine, 4% % valine, 0.2% glacial acetic acid, 3% collagen, 2% glycosaminoglycan, 0.3% retinoic acid and 0.8% ethanolamine; the balance is normal saline.
Embodiment 2
[0035] A hepatic stem cell injection for treating diabetes, each ml injection mixture contains 3×10 5 hepatic stem cells and 1×10 4 umbilical cord mesenchymal stem cells; the injection mixture consists of the following mass percentage components: 8% polyethylene glycol, 5% arginine, 7% lysine, 6% methionine, 7% % valine, 0.8% glacial acetic acid, 5% collagen, 5% glycosaminoglycan, 0.3-0.5% retinoic acid and 1.2% ethanolamine; the balance is normal saline.
[0036] Preparation:
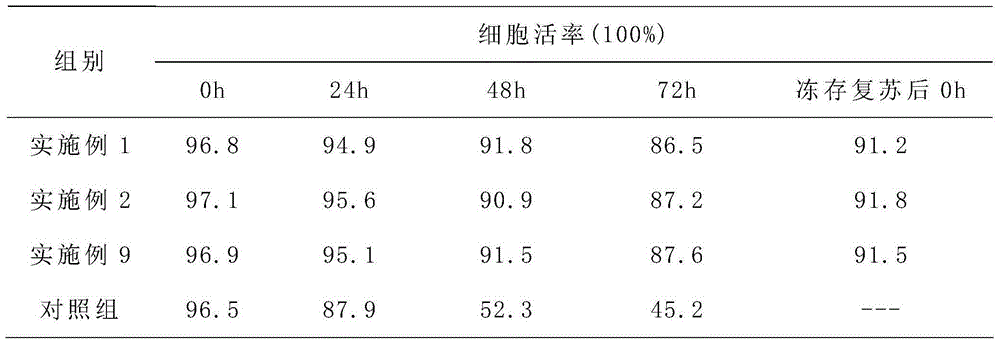
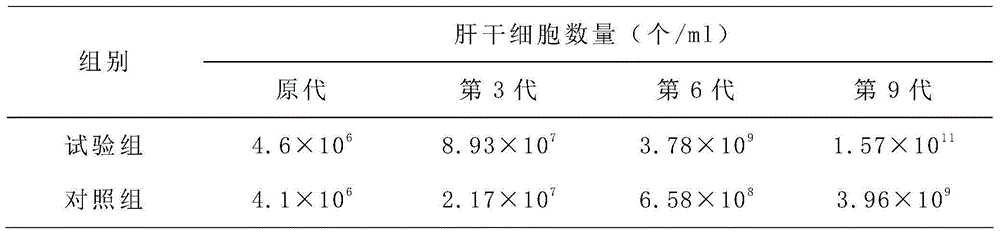
[0037] 1) Culture of liver stem cells;
[0038] 2) Culture of umbilical cord mesenchymal stem cells;
[0039] 3) Polyethylene glycol, arginine, lysine, methionine, valine, glacial acetic acid, collagen, glycosaminoglycan, retinoic acid and ethanolamine are dissolved in normal saline to prepare a compound solution, Pre-cool at 4°C for later use;
[0040] 4) Resuspend the hepatic stem cells obtained in step 1) and the umbilical cord mesenchymal stem cells obtained in step 2) in the compound solution...
Embodiment 3
[0042] A hepatic stem cell medium, based on DMEM / F12 medium, comprising the following components at concentrations: 6mg / ml hyaluronic acid, 60ng / ml hepatocyte growth factor, 50ng / ml basic fibroblast growth factor, 10μg / ml ml recombinant human bone morphogenetic protein, 300 μg / ml 2′,3′-dideoxyadenosine-5-triphosphate, 7 mg / ml ferric citrate, 10 mg / ml carboxymethylcellulose sodium and 3 mg / ml L-carnitine .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com