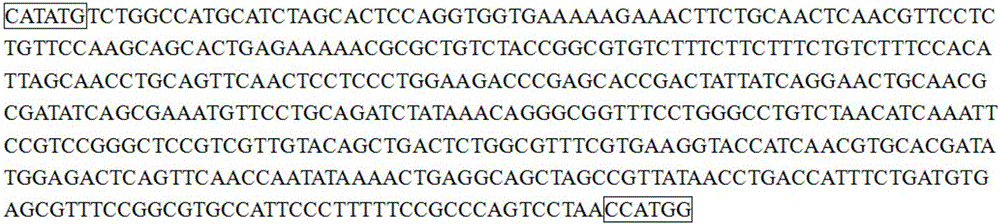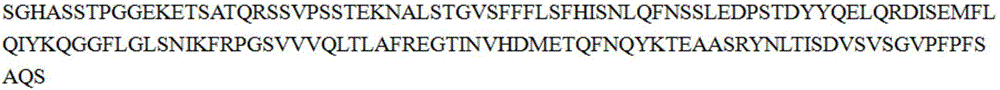Tumor antigen protein and tumor vaccine
A tumor antigen and tumor vaccine technology, which is applied in the direction of tumor-specific antigen, tumor rejection antigen precursor, anti-tumor drugs, etc., can solve the problems of poor therapeutic effect and poor specificity of peptide vaccines, and achieve good therapeutic effect and good therapeutic effect. Safety and efficacy, effect of increasing action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 prepares MCMVax vaccine
[0041] 1. MUC1-Xex gene design and synthesis
[0042] The MUC1-Xex coding gene was designed using DNA design software, the coding gene was codon optimized, and an NdeI endonuclease site was introduced at its 5' end, and an NcoI endonuclease site was introduced at the 3' end, as shown in SEQ ID NO:4 , The synthetic gene was cloned into the pUC57 vector, ampicillin resistance.
[0043] 2. Enzyme digestion and sequencing identification of genes
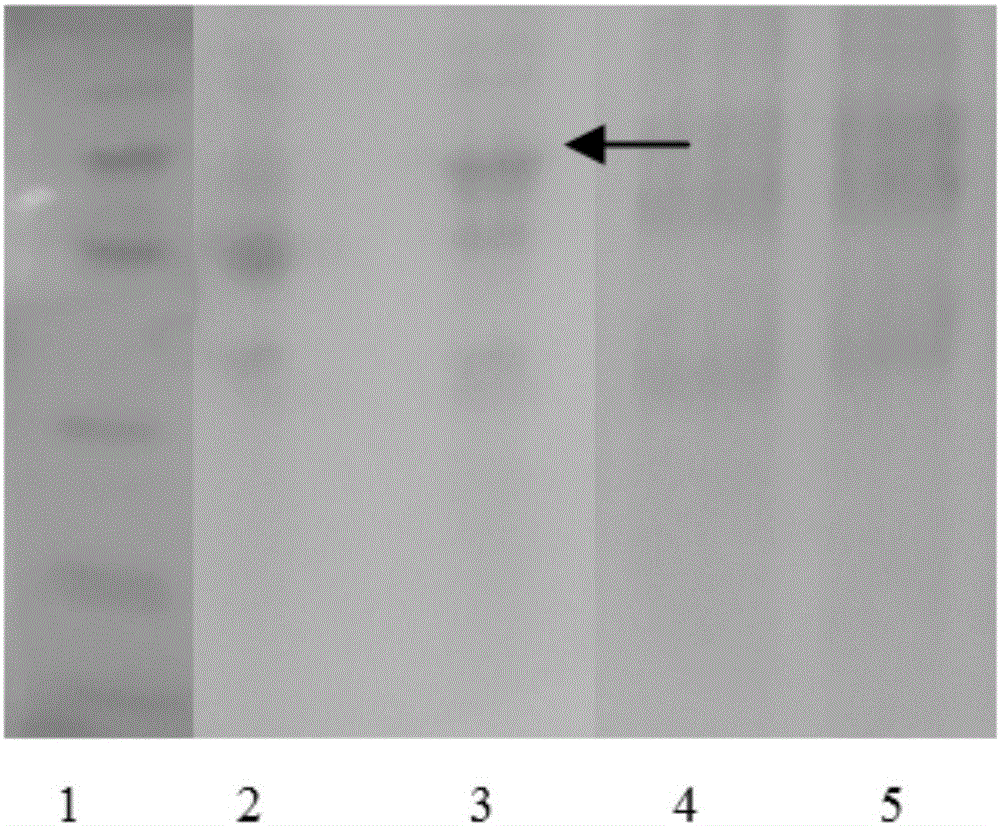
[0044] After gene synthesis, double digestion was performed with NdeI and NcoI endonucleases, ligated into the pMAL-p5X plasmid that had undergone the same digestion, ligated with DNA ligase, and transformed into competent DH5α bacteria. Screen on agar culture plates containing ampicillin (AMP), select single clones and culture them in LB-AMP medium (i.e. LB medium containing AMP), extract plasmids and identify them, and then send them to Beijing Tianyi Huiyuan Biotechnology Co., Ltd. Co.,...
Embodiment 2
[0053] Example 2 The inhibitory effect of MCMVax on melanoma
[0054] Fifteen male C57BL / 6J mice aged 6-8 weeks were divided into three groups: control group, MNRVax treatment group and MCMVax treatment group, with 5 mice in each group, and B16-Muc1 melanoma cells were inoculated subcutaneously in the right armpit of each mouse for 2.8 ×10 6 One, the tumor was visible after 5 days.
[0055] In the control group, 100 μL of normal saline was injected into the hind leg muscles of each mouse, twice a week, for a total of 4 injections;
[0056] In the MNRVax treatment group, 50 μg of MNRVax was injected into the hind leg muscle of each mouse, twice a week, for a total of 4 injections;
[0057] In the MCMVax treatment group, 50 μg of MCMVax was injected into the hind leg muscle of each mouse, twice a week, for a total of 4 times.
[0058] The results showed that compared with the mice in the control group, the tumors of the mice in the MCMVax treatment group were significantly re...
Embodiment 3
[0062] Example 3 The inhibitory effect of MCMVax combined with MNRVax on melanoma
[0063] Twenty-four male C57BL / 6J mice aged 6-8 weeks were divided into four groups: control group, MNRVax treatment group, MCMVax treatment group, MNRVax and MCMVax combined treatment group, 6 mice in each group, and inoculated subcutaneously in the right armpit of each mouse B16-Muc1 melanoma cells 2.4×10 6 One, the tumor was visible after 9 days.
[0064] The formulations were injected into the hind leg muscles of the mice, 3 times a week, 3 times in total. Among them, the control group was injected with 200 μL of normal saline each time; the MNRVax treatment group was injected with 50 μg MNRVax each time; the MCM Vax treatment group was injected with 50 μg MCMVax each time; the combined treatment group was injected with 50 μg MNRVax plus 50 μg MCMVax each time.
[0065] The results showed that compared with the mice in the control group, the tumors of the mice in the MCMVax treatment group w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com