Compositions of human prothrombin and activated factor X for improving hemostasis in the treatment of bleeding disorders
A technology of human prothrombin and prothrombin, which is applied in the field of the composition of human prothrombin and activated factor X for improving hemostasis in the treatment of bleeding diseases, which can solve the problems of many side effects and not being equally effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The production of recombinant proteins such as FII and FXa is well known in the art. For example, EP 1460131 describes a method for the production of recombinant human coagulation factors in human cell lines. Further, WO 2005 / 038019 describes the recombinant production of coagulation factors in high yields. Methods for isolating coagulation factors from plasma preparations are also well known in the art. For example, US Patent 4,883,598 describes a method for separating coagulation factors from plasma or plasma products using liquid chromatography.
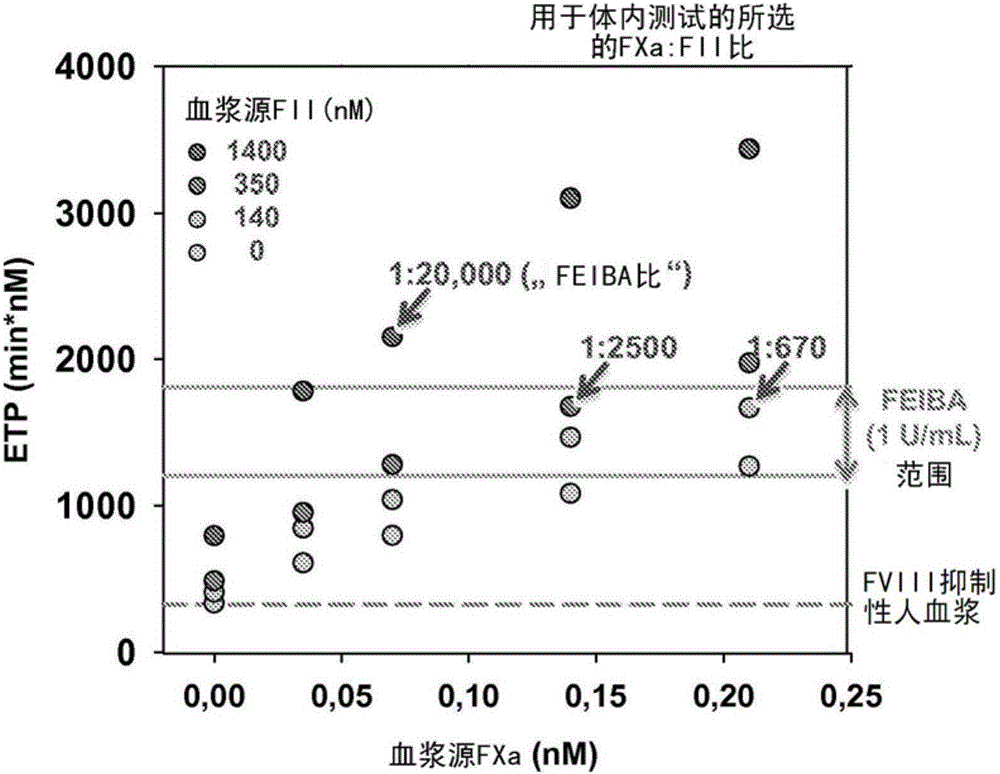
[0027] is a plasma-derived activated prothrombin complex concentrate used in the treatment of bleeding conditions in hemophiliacs with inhibitors. FII and FXa are thought to mediate The most critical ingredient for the hemostatic effect. exist The molar ratio of FXa to FII is in the range of 1:20,000-40,000, that is, 1U / mL Produced plasma concentrations of 0.035-0.07 nM FXa and 1400 nM FII. A range of molar rati...
Embodiment 1
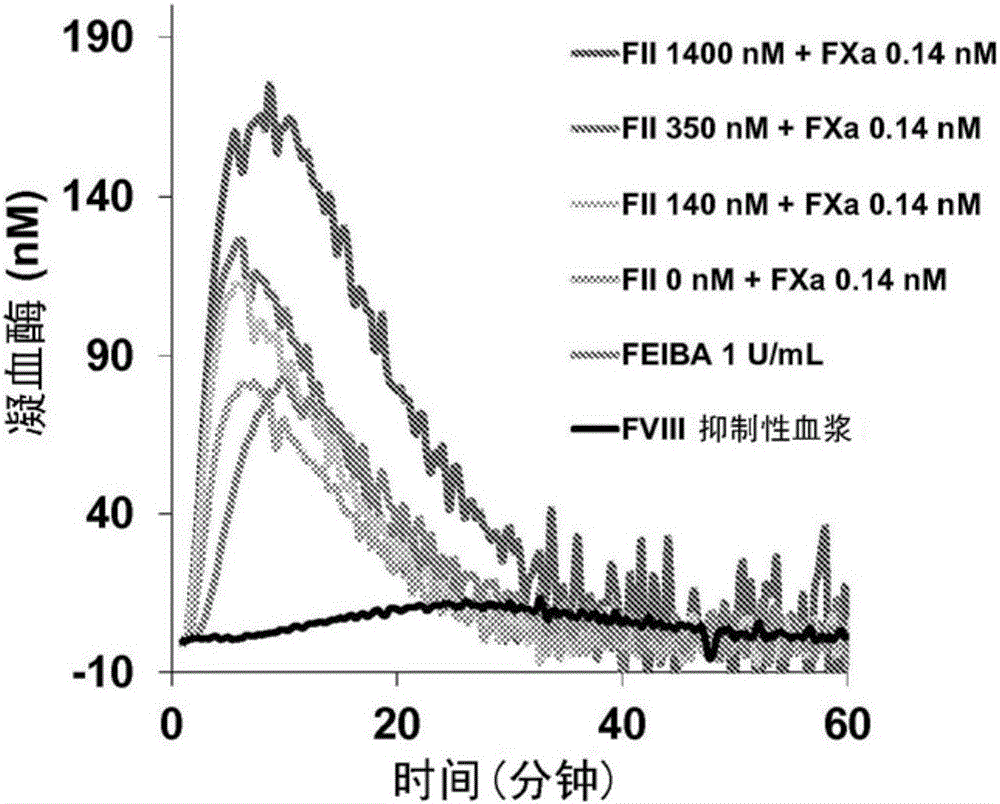
[0047] Example 1: In vitro evaluation of the FXa / FII combination in a thrombin generation assay
[0048] The pro- and anticoagulant effects of substances in human plasma were monitored using the AutoCorrect Thrombin Curve (CAT) assay. The thrombin curve (thrombogram) represents the concentration of thrombin in coagulated plasma, so CAT is a general physiological function test of the hemostatic system. The assay is based on the measurement of fluorescence from cleavage of the fluorogenic substrate Z-G-G-R-AMC by thrombin over time with tissue factor (TF)-initiated coagulation. The measurement was performed on a 96-well plate fluorometer Thrombograph purchased from Thermo Scientific TM implementation, and a thrombin calibrator must be used to correct for internal filter effects, differences in plasma color from donor to donor, substrate elimination, and instrument variability.
[0049] The procoagulant activity of different combinations of plasma-derived FII (140-1400 nM) an...
Embodiment 2
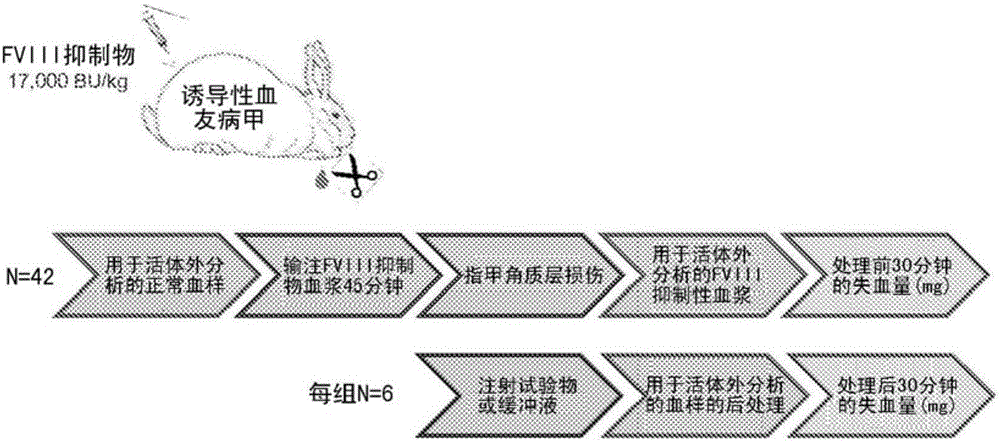
[0057] Example 2: In Vivo: Proof of Concept for Efficacy in Induced Hemophilia Rabbit Model
[0058] The purpose of this proof-of-concept study was to evaluate the efficacy of three FII / FXa doses administered together or alone in a nail-clip bleeding model in FVIII-inhibited rabbits ( Figure 3A ). The dose with the highest FII content is 4.4mg / kgFII+275ng / kg FXa, which is defined as " Ratio", both FII and FXa were defined as 100%. In the second dose of 1.1 mg / kg FII+551 ng / kg FXa, FII was reduced to 25% and FXa was increased to 200%. The third dose was 0.44 mg In FII / kg FII+826ng / kg FXa, FII was reduced to 10% and FXa was increased to 300%. For each treatment, efficacy was determined as the reduction in post-treatment blood loss compared to the buffer-only control group. by (75U / kg) as a positive control.
[0059] Conscious New Zealand Great White (NZW) rabbits were intravenously administered 2 mL / kg of FVIII inhibitor (17,000 BU / kg) to deplete endogenous FVIII. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com