Oral aripiprazole liquid dry suspension agent and preparation method thereof
A technology of aripiprazole and dry suspension, which is applied in the field of medicine and can solve the problems of harm to patients, inconvenient administration, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
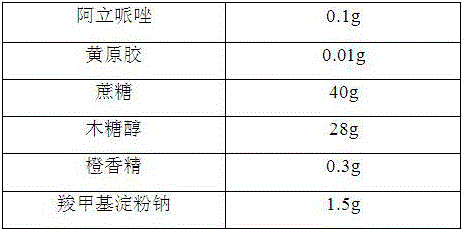
[0013]
[0014] Preparation Process:
[0015] 1) Grinding the aripiprazole, sucrose and xylitol through a 100-mesh sieve for later use;
[0016] 2) Mix the aripiprazole, the above-mentioned sucrose, xylitol, and xanthan gum evenly, add a binder, stir and granulate, dry at 50°C, granulate, and classify to obtain granules for later use;
[0017] 3) Mix the flavoring agent, sodium carboxymethyl starch and the granules prepared in the above 2).
Embodiment 2
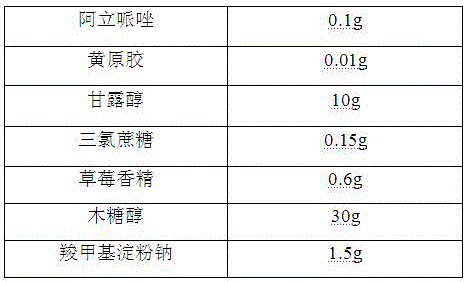
[0019]
[0020] Preparation Process:
[0021] 1) Pass the aripiprazole, mannitol, and xylitol through a 100-mesh sieve for later use;
[0022] 2) Mix the aripiprazole with the above-mentioned mannitol, xylitol, and xanthan gum evenly, add a binder, stir and granulate, dry at 50°C, granulate, and classify to obtain granules for later use;
[0023] 3) Mix sucralose, strawberry essence, sodium carboxymethyl starch and the granules prepared in the above 2).
Embodiment 3
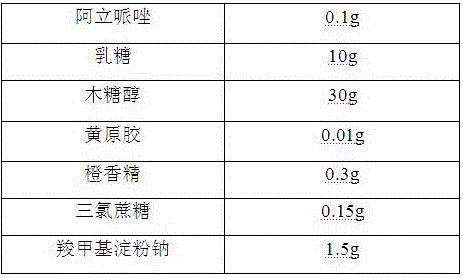
[0025]
[0026] Preparation Process:
[0027] 1) Pass the aripiprazole, lactose, and xylitol through a 100-mesh sieve for later use;
[0028] 2) Mix the aripiprazole with the above-mentioned lactose, xylitol, and xanthan gum evenly, then add a binder, stir and granulate, dry at 50°C, granulate, and classify to obtain granules for later use;
[0029] 3) Mix sucralose, orange essence, sodium carboxymethyl starch and the granules prepared in 2) above to get the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com