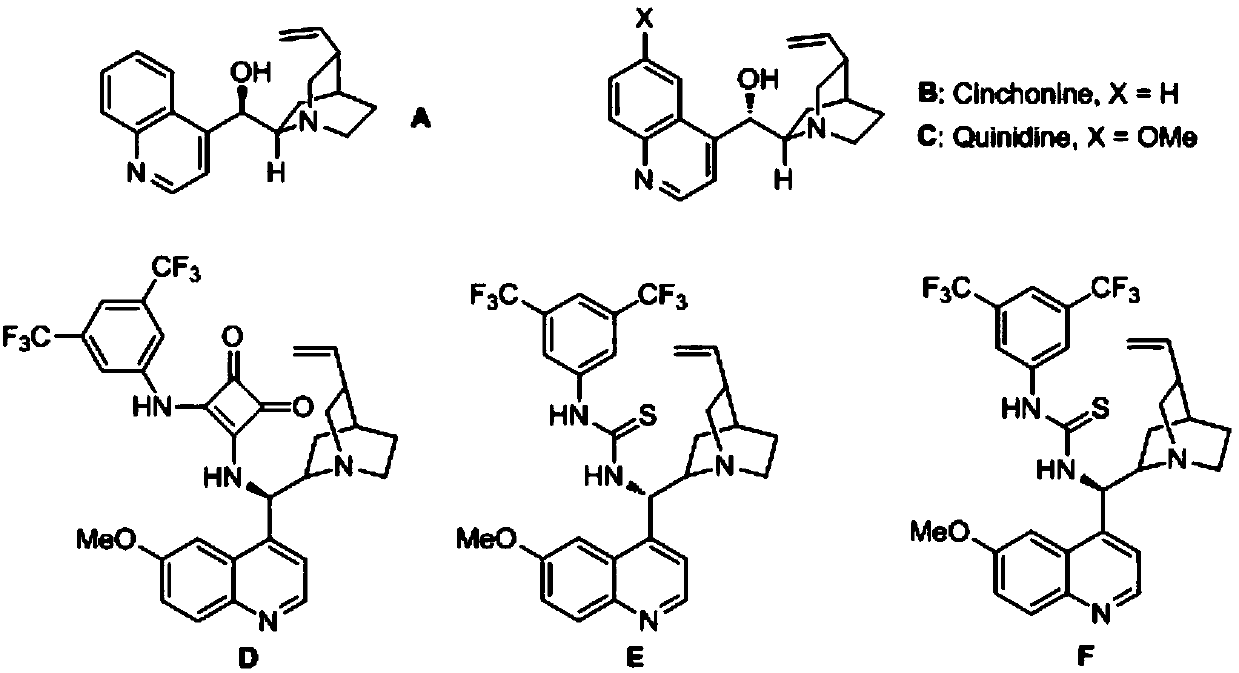
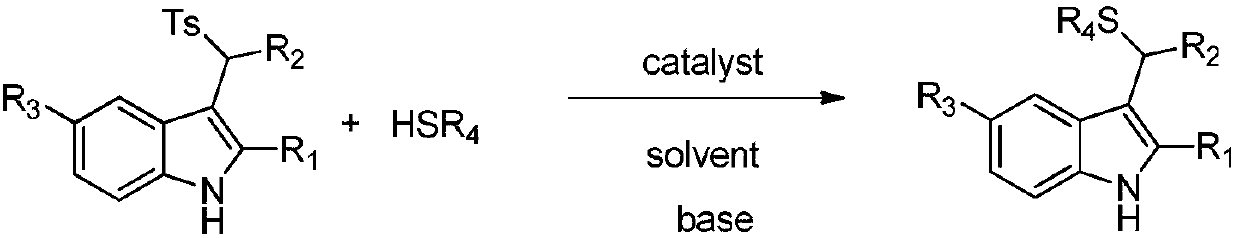
Application of chiral organic bases in preparation of chiral sulfur-containing indole compounds in water-oil two-phase system
A technology for indole compounds and organic bases to catalyze mercaptans, applied in organic compound/hydride/coordination complex catalysts, organic chemistry, organic chemical methods, etc., to achieve high reactivity and enantioselectivity, and sulfuration reaction conditions Gentle, easy-to-prepare effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: optimization of conditions
[0034] In the reaction vessel, drop into the chiral organic base catalyst (10mol% of the amount of substrate 1a in formula 1), add two times the equivalent of inorganic base, then add p-toluenesulfonyl indole and triphenylmercaptan, to this mixture After adding 0.5 ml of organic solvent to dissolve, add water. The reaction system was reacted at a specific temperature. After the reaction, saturated brine twice the volume of the reaction system was added, extracted with ethyl acetate, and the organic phases were combined. After removing the solvent, the pure product was directly separated by column chromatography. The reaction formula and catalyst structure are as follows:
[0035]
[0036] The conversion rate was determined from the reaction crude product with 1,3,5-trimethoxybenzene as the internal standard 1 H NMR to determine, the enantiomeric excess of the product was determined by chiral liquid chromatography, see Tabl...
Embodiment 2
[0044] Example 2: Synthesis of sulfur-containing indole compound 3a in water-oil two-phase system
[0045] Drop into quinine thiourea alkaloid (F) in reaction vessel as catalyst (10mol% of substrate 1a consumption in formula 1), then add the sodium carbonate of 2 times equivalent (substrate 1a), then add 0.2mmol p-formazol phenylsulfonylindole (R 1 =CH 3 , R 2 = Ph, R 3 =H) and 0.22 mmol triphenylmercaptan, after adding 200 microliters of chloroform to dissolve the mixture, and then adding 4 milliliters of water. The reaction system was reacted at a temperature of zero degrees Celsius, and the reaction time was 12 hours. After the reaction was completed, the saturated saline twice as much as the reaction system was added, and then extracted with ethyl acetate, and the organic phase was combined. After removing the solvent, it was directly separated by column chromatography. The pure product 94.2 mg of 3a was obtained in 95% yield with an enantioselectivity of 91%.
Embodiment 3
[0046] Example 3: Synthesis of sulfur-containing indole compound 3b in water-oil two-phase system
[0047] Operation steps are with embodiment 3a, wherein (R 1 = Me,R 2 =4-ClC 6 h 4 , R 3 =H), the reaction time was 12h, and 101.8mg of 3b was obtained with a yield of 96% and an enantioselectivity of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com