Method for synthesizing cyclic peptides through enzyme method
A cyclic peptide and enzymatic activity technology, applied in the field of enzymatic synthesis of cyclic peptides, can solve the problems of complex steps, toxic and side effects, unfavorable to environmental protection, etc., and achieve the effects of high yield and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The design of embodiment 1 linear peptide
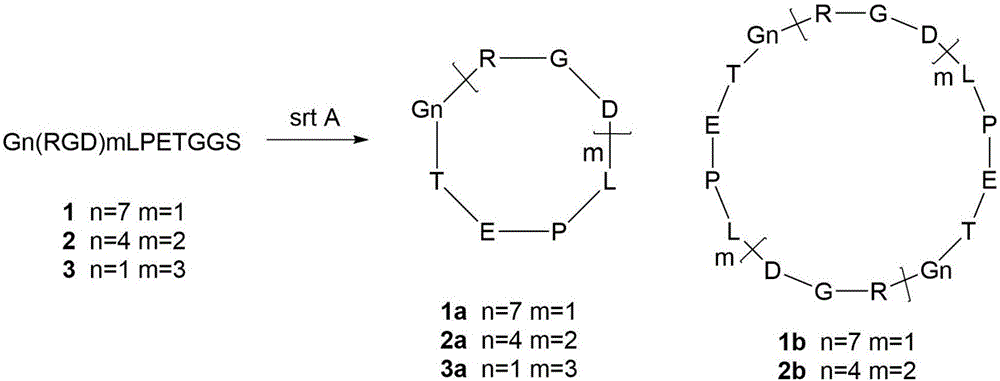
[0016] (1) For the linear peptide containing the RGD sequence, design a polypeptide sequence containing 17 amino acids, including the sortaseA recognition sequence LPETGGS at the C segment and the polyglycine sequence at the N-terminal, which is convenient for sortaseA to recognize and cyclize, and the sequence contains 1-3 RGD sequence, multivalent RGD in order to improve the biological activity of the peptide; the prepared linear peptide sequence is Gn(RGD)mLPETGGS; where n=7, m=1; or n=4, m=2; or n=1, m = 3; the synthetic linear peptide was able to inhibit the interaction of integrin αvβ3 with the extracellular matrix.
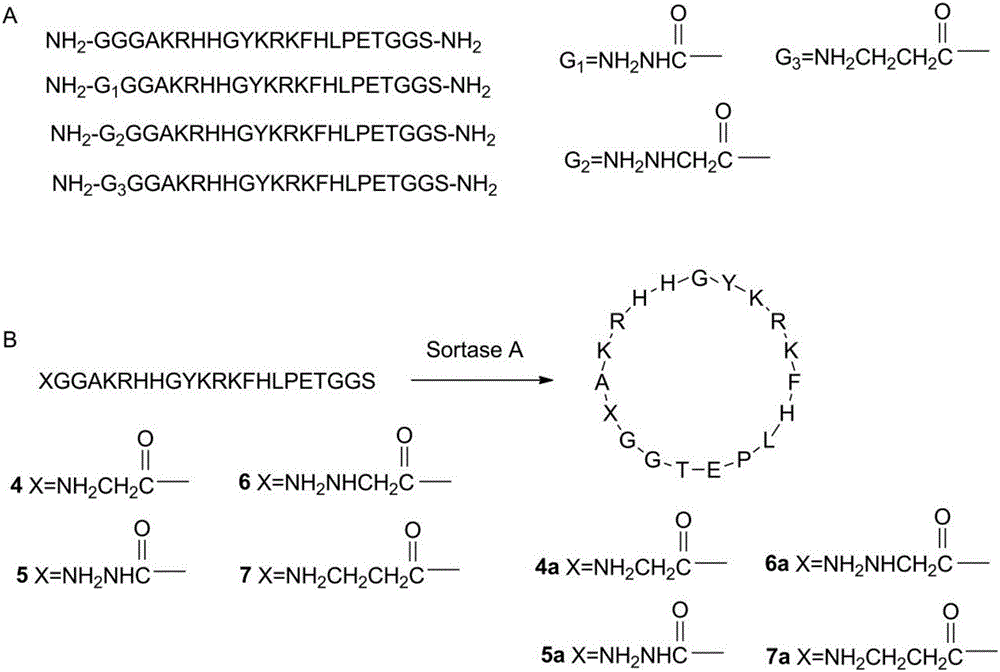
[0017] (2) For the linear peptide containing the antimicrobial peptide AKRHHGYKRKFH, the sortase A recognition sequence LPETGGS is carried on the C segment of the antimicrobial peptide, and the N segment is a polyglycine sequence, and the last glycine in the polyglycine sequence is structurally modified. G...
Embodiment 2
[0018] Example 2 The linear peptide G7RGDLPETGGS synthesizes RGD cyclic peptide under the catalysis of sortaseA enzyme
[0019] Linear peptide synthesis: Weigh Fmoc-Ser(tBu)-Wang resin and swell overnight in dimethylformamide (DMF) solution, remove Fmoc with 20% (V / V) piperidine, and dichloromethane (DCM) , MeOH and DMF to clean the resin, take a small amount of resin and detect it with ninhydrin chromogenic method, and heat it to 100°C to detect that the resin turns blue, indicating that Fmoc has been removed completely. 5eq (resin is 1eq) of amino acid, 5eq condensing agent O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroborate (TBTU) and 10eq diisopropyl ethyl Amine (DIPEA) was dissolved in DMF, added to the solid-phase synthesis tube and reacted for 2 hours, followed by washing the resin with DCM, MeOH, and DMF, taking a small amount of resin and using the ninhydrin color method to detect the yellow color of the resin, indicating that the free amino group and amino...
Embodiment 3
[0021] Example 3 Linear peptide G4 (RGD) 2LPETGGS synthesizes RGD cyclic peptide under the catalysis of sortaseA enzyme
[0022] Linear peptide synthesis: the method is the same as Example 1
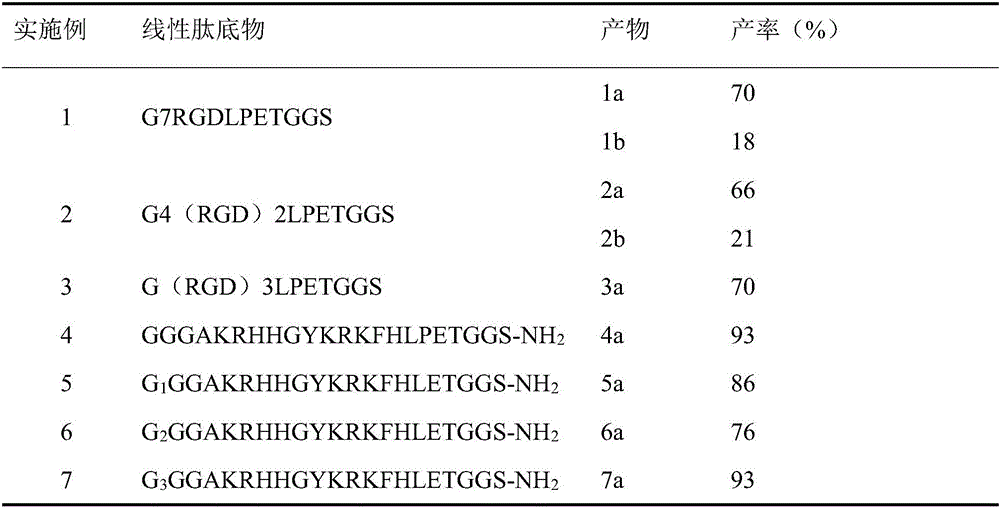
[0023] Enzyme-catalyzed synthesis of cyclic peptides: in 100 μL enzyme reaction system, take linear peptide G4(RGD)2LPETGGS 0.25 mM; sortase A 10 μM; reaction buffer is 0.3M Tris-HCl (PH=7.5), 0.15M NaCl, 5mM CaCl 2 , 2mM mercaptoethanol, reacted at 37°C for 20h. After the reaction, the enzyme was filtered by ultrafiltration, and the resulting filtrate was purified by HPLC and identified by mass spectrometry, and HPLC gradient elution: mobile phase A was eluted from 5% to 50% for 20 minutes; the molecular weight of the cyclic peptide obtained by the G4(RGD)2LPETGGS reaction was respectively 1325.4, 2650.7, which are characterized by inhibiting the interaction between integrin αvβ3 and extracellular matrix. The yields are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com