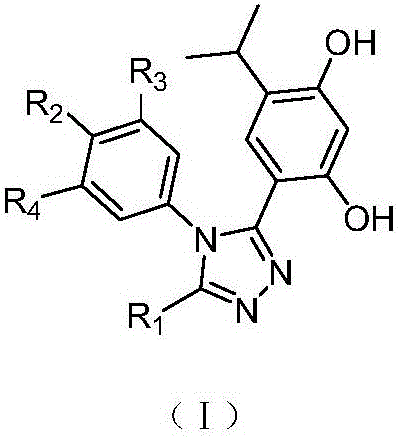
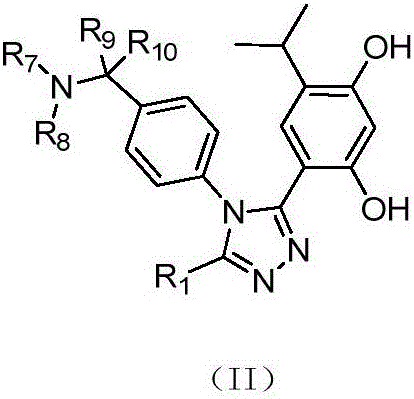
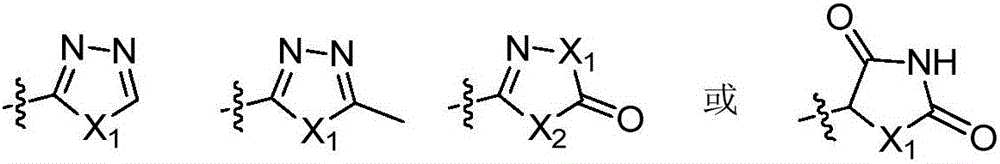
3,4-diphenyl-4H-1,2,4-triazole derivative as well as preparation method and application thereof
A technology of triazole derivatives and diphenyl, applied in the field of drug synthesis, can solve problems such as toxicity and prevention of clinical development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] 5-(2,4-diphenolhydroxy-5-isopropylbenzene)-N-ethyl-4-(4-methyl-2,3,4,5-tetrahydrobenzo[b][1, 4] Oxazepine-8-yl)-4H-1,2,4-triazole-3-carboxamide
[0119]
[0120] Step 1: 2-(Hydroxymethyl)-5-nitrophenol
[0121] Compound 2-hydroxyl-4-nitrobenzoic acid (15g, 82mmol) was added to dry tetrahydrofuran (100mL), and the concentration of 10M borane dimethyl sulfide solution (82mL, 820mmol) was slowly added dropwise under ice bath, and the dropwise addition was completed Afterwards, the temperature was raised to room temperature and reacted for 16 h. Cool to 0°C, slowly add methanol dropwise to quench. The temperature was raised to 70° C. and refluxed for 1 h. After concentration, ethyl acetate was added, washed with brine, dried and concentrated to obtain 2-(hydroxymethyl)-5-nitrophenol (13.5 g) with a yield of 97.5%.
[0122] MS m / z (ESI): 168.1.
[0123] Step 2: 2-Hydroxy-4-nitrobenzaldehyde
[0124] Compound 2-(hydroxymethyl)-5-nitrophenol (13g, 77mmol) and manganese...
Embodiment 2
[0150] 4-(4-Cyclohexyl-3-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-8-yl)-5-(2,4- Diphenol hydroxy-5-isopropylphenyl)-N-ethyl-4hydro-1,2,4-triazole-3-carboxamide
[0151]
[0152] Step 1: 2-((cyclopropylamine)methyl)-5-nitrophenol
[0153] The compound 2-hydroxy-4-nitrobenzaldehyde (1.8 g, 10.8 mmol) was dissolved in methanol (50 mL), and cyclopropylamine (800 mg, 14 mmol) and acetic acid (650 mg, 10.8 mmol) were added. After reacting at room temperature for 0.5h, sodium cyanoborohydride (1.35g, 21.6mmol) was added in batches, and the reaction was continued for 3h. After concentration, dichloromethane was added, washed with saturated sodium bicarbonate and brine, dried and concentrated to obtain 2-( (Cyclopropylamine)methyl)-5-nitrophenol (2.1 g) yield 93.6%.
[0154] MS m / z (ESI): 209.2.
[0155] Step 2: 2-Chloro-N-cyclopropyl-N-(2-hydroxy-4-nitrobenzyl)acetamide
[0156] The compound 2-((cyclopropylamine) methyl)-5-nitrophenol (1.6g, 7.7mmol) and triethylamine (2.2mL...
Embodiment 3
[0172] 5-(2,4-diphenolhydroxy-5-isopropylbenzene)-N-(3-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepine (Zen-8-yl)-N-ethyl-4hydro-1,2,4-triazole-3-carboxamide
[0173]
[0174] Step 1: 2-(((2,4-dimethoxybenzyl)amino)methyl)-5-nitrophenol
[0175] The compound 2-hydroxyl-4-nitrobenzaldehyde (4g, 23.9mmol) was dissolved in methanol (100mL), and 2,4-dimethoxybenzylamine (4.8g, 28.7mmol) and acetic acid (1.44g, 23.9 mmol). After reacting at room temperature for 0.5h, sodium cyanoborohydride (3g, 47.8mmol) was added in batches, and the reaction was continued for 2h. After concentration, dichloromethane was added, washed with saturated sodium bicarbonate and saturated brine, dried and concentrated, and the crude product was beaten (dichloro Methane / petroleum ether=1:10) gave 2-(((2,4-dimethoxybenzyl)amino)methyl)-5-nitrophenol (6.5 g) with a yield of 85.3%.
[0176] MS m / z (ESI): 319.1.
[0177] Step 2: 2-chloro-N-(2,4-dimethoxybenzyl)-N-(2-hydroxy-4-nitrobenzyl)acetamide
[017...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com