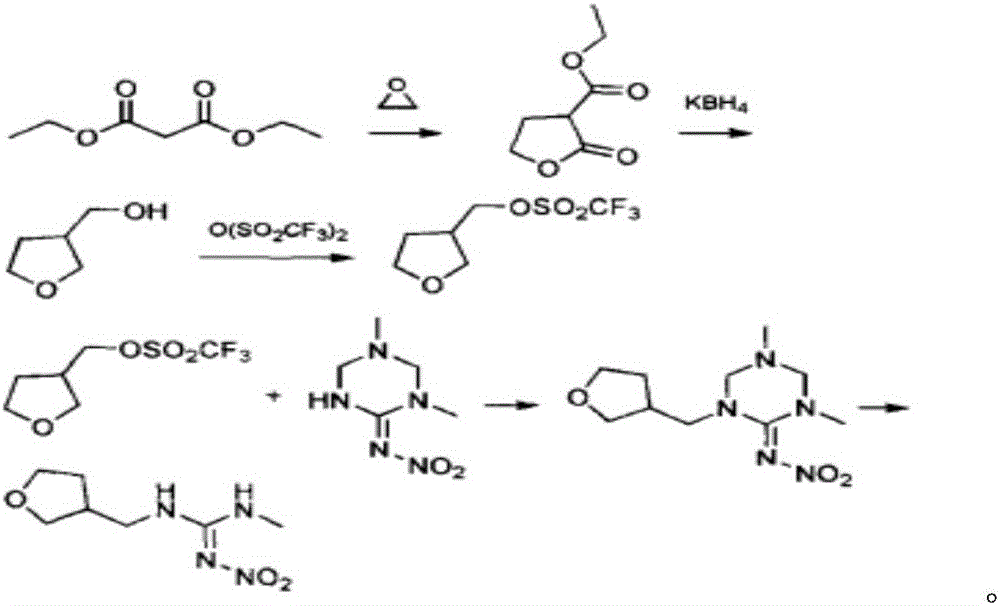
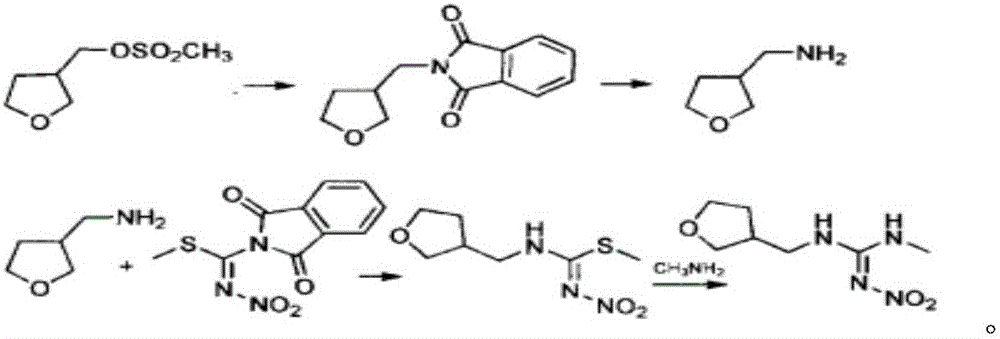
Synthesis method of dinotefuran
A synthetic method, the technology of dinotefuran, is applied in the field of neonicotinoid insecticide synthesis, which can solve the problems of high toxicity of dimethyl sulfate, safety risks, and complicated steps, so as to ensure the ecological environment and food safety, and avoid production The effect of dangerous operation and significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A synthetic method for dinotefuran, comprising the following steps:
[0046] 1) Aldol condensation and reduction:
[0047]
[0048] In a 250mL three-necked round-bottom flask, dissolve 10g of sodium methoxide and 15g of γ-butyrolactone in 100g of n-hexane, stir for 1h until they are completely dissolved, then control the temperature at 10°C, and feed 10g of formaldehyde gas within 2h, then Insulate and react at 30°C for 8 hours, take samples to determine the normalized content of γ-butyrolactone ≤ 0.5%, the aldol condensation reaction is completed, remove the residual formaldehyde gas in the n-hexane solution under low temperature and negative pressure, neutralize it with hydrochloric acid to weak acidity, After filtering, the solvent was distilled off from the filtrate under negative pressure to obtain 18.5 g of a-hydroxymethyl-γ-butyrolactone with a content of 98% and a yield of 90%.
[0049] Dissolve 18.5g of a-hydroxymethyl-γ-butyrolactone in 60mL of tetrahydrofur...
Embodiment 2
[0064] A synthetic method for dinotefuran, comprising the following steps:
[0065] 1) Aldol condensation and reduction:
[0066]
[0067] In a 250mL three-neck round bottom flask, dissolve 10g sodium methoxide and 15g γ-butyrolactone in 100g n-hexane, stir for 3 hours until they are completely dissolved, then control the temperature at 50°C, and feed 10g of formaldehyde gas within 4 hours, then Insulate and react at 60°C for 13 hours, take samples to determine the normalized content of γ-butyrolactone ≤ 0.5%, after the aldol condensation reaction is completed, remove the residual formaldehyde gas in the n-hexane solution under low temperature and negative pressure, neutralize it with hydrochloric acid to weak acidity, After filtering, the solvent was distilled off from the filtrate under negative pressure to obtain 18.5 g of a-hydroxymethyl-γ-butyrolactone with a content of 97% and a yield of 91%.
[0068] Dissolve 18.5g of a-hydroxymethyl-γ-butyrolactone in 60mL of tetra...
Embodiment 3
[0083] A synthetic method for dinotefuran, comprising the following steps:
[0084] 1) Aldol condensation and reduction:
[0085]
[0086] In a 250mL three-necked round-bottom flask, dissolve 10g of sodium methoxide and 15g of γ-butyrolactone in 100g of n-hexane, stir for 2 hours until they are completely dissolved, then control the temperature at 30°C, and feed 10g of formaldehyde gas within 3 hours, then Insulate and react at 40°C for 10 hours, take samples to determine the normalized content of γ-butyrolactone ≤ 0.5%, after the aldol condensation reaction is completed, remove the residual formaldehyde gas in the n-hexane solution under low temperature and negative pressure, neutralize it with hydrochloric acid to weak acidity, After filtration, the solvent was distilled off from the filtrate under negative pressure to obtain α-hydroxymethyl-γ-butyrolactone, 18.5 g, content 96%, yield 91%.
[0087] Dissolve 18.5g of a-hydroxymethyl-γ-butyrolactone in 60mL of tetrahydrofu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com