Recombinant expression vector, host and construction method thereof, and animal model construction method
An expression vector and host technology, which is applied in the field of recombinant expression vector and animal model construction, can solve the problems of failure to successfully establish pre-existing anti-MICA antibody animal models, no observation of changes in lymphocytes, difficulty in purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of humanized soluble HLA-A2 protein
[0055] (1) Human THP-1 cell culture
[0056] Human THP-1 cells were placed in 1640 medium containing 10% fetal bovine serum at 37°C, 5% CO 2 Incubate in an incubator, replace the culture medium every three days, and culture the cells to the logarithmic phase.
[0057] (2) Design and synthesis of primers
[0058] According to the HLA-A2 sequence published on GenBank, a pair of primers were designed by using Olig6.0 software to amplify the mature peptide gene sequence of HLA-A2 extracellular segment (AF1, AR1). Company Synthesis.
[0059] AF1:5'CGA ACC CTC GTC CTG CTA 3'
[0060] AR1:5'GCT CTT CCT CCT CCA CAT CA 3'
[0061] (3) Extraction of total RNA from THP-1 cells
[0062] 1. After the human THP-1 cells are separated and collected, put them into a 1.5ml EP tube, wash once with 1×PBS, add 1ml Trizol to fully dissolve, and blow evenly with a sampler to completely dissociate the nucleoprotein from the nucl...
Embodiment 2
[0137] Example 2 Construction of HLA-A2 expression vector and expression of target protein
[0138] (1) Design and synthesis of primers
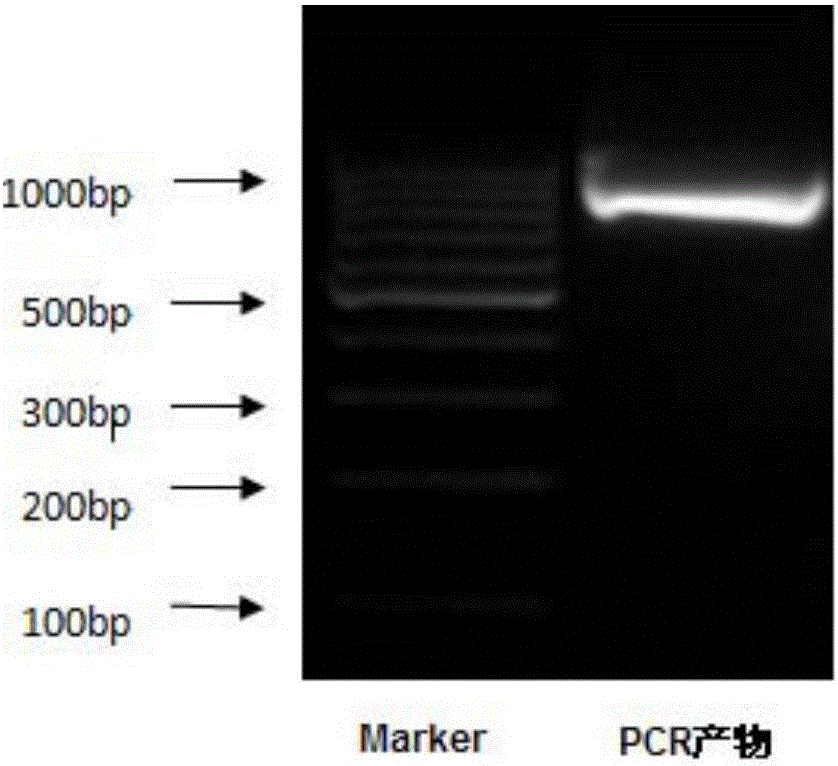
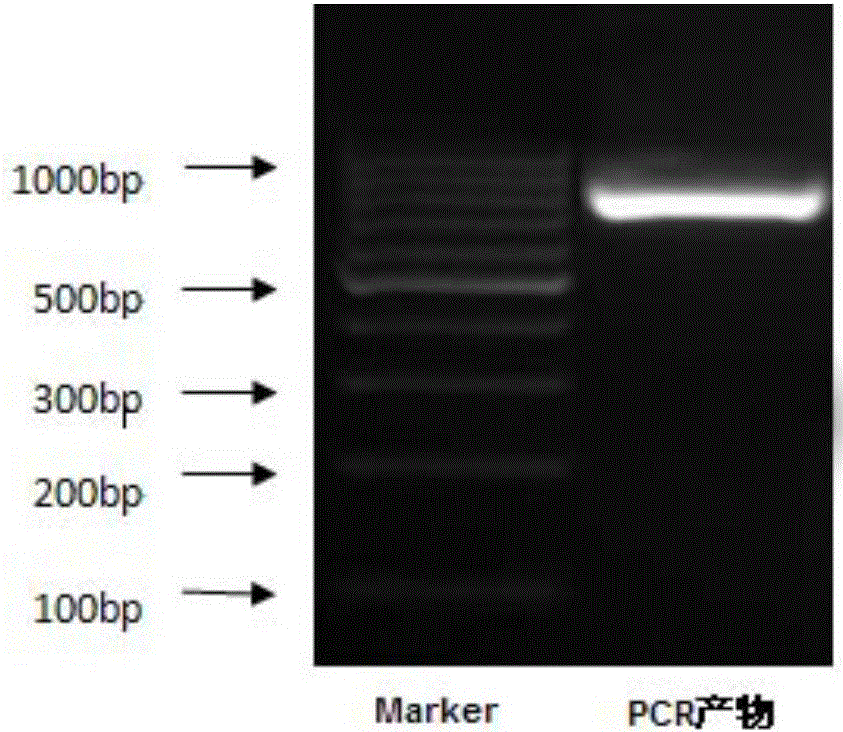
[0139] 1. Use the mature peptide gene sequence of the extracellular region of the recombinant plasmid PMD19-HLA-A2 as a template to design specific primers to amplify the target gene sequence. The primers were synthesized by Nanjing Zhongding Co., Ltd. The yellow mark is the recombination arm, the red is the restriction site, and the underlined part is the designed primer sequence (the sequence of the upstream primer is shown in SEQ ID No.3, and the sequence of the downstream primer is shown in SEQ ID No.4), see Figure 17 . in Figure 17 (A) shows the upstream primer HLA-A2-F, Figure 17 (B) shows the downstream primer HLA-A2-R.
[0140] (2) Construction of pCzn1-HLA-A2 recombinant plasmid
[0141] 1. PCR amplification and recovery of HLA-A2 extracellular segment gene
[0142] Using the PMD19-HLA-A2 recombinant cloning plasmid as a te...
Embodiment 3
[0247] Example 3 Constructing a BALb / c mouse model with pre-existing anti-HLA-A2 antibodies
[0248] 1. Obtain soluble anti-HLA-A2 protein with a protein concentration of 0.49 mg / ml through a prokaryotic expression system, mix 25 μg, 50 μg, 100 μg, and 200 μg of HLA-A2 protein with an equal volume of Freund’s complete adjuvant, and apply to the abdominal wall of the mouse Subcutaneous injection. At 1 week, 2 weeks, 4 weeks, 6 weeks, and 8 weeks, the inner canthus vein of the eyeball was collected, about 200-300 μl was placed into a coagulation-promoting tube, and the serum was collected by centrifugation. The ELISA (enzyme-linked immunosorbent assay) method was used to detect the Antibody to HLA-A2. Four different doses of HLA-A2 protein can make the peripheral blood of BALb / c mice produce anti-HLA-A2 antibody, the antibody began to increase at 1 week, the antibody concentration reached the peak at 4 weeks, and then the antibody gradually decreased, especially at 25 μg and 50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com