Preparation method for catalytic synthesis of (R)-4-cyano-3-hydroxybutanoate by halohydrin dehalogenases
A technology of ethyl hydroxybutyrate and halohydrin dehalogenase, applied in fermentation and other directions, can solve the problems of low utilization rate, long residence time, and disintegration, etc., so as to improve the utilization rate of enzymes, shorten the residence time, and reduce the reaction time. mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
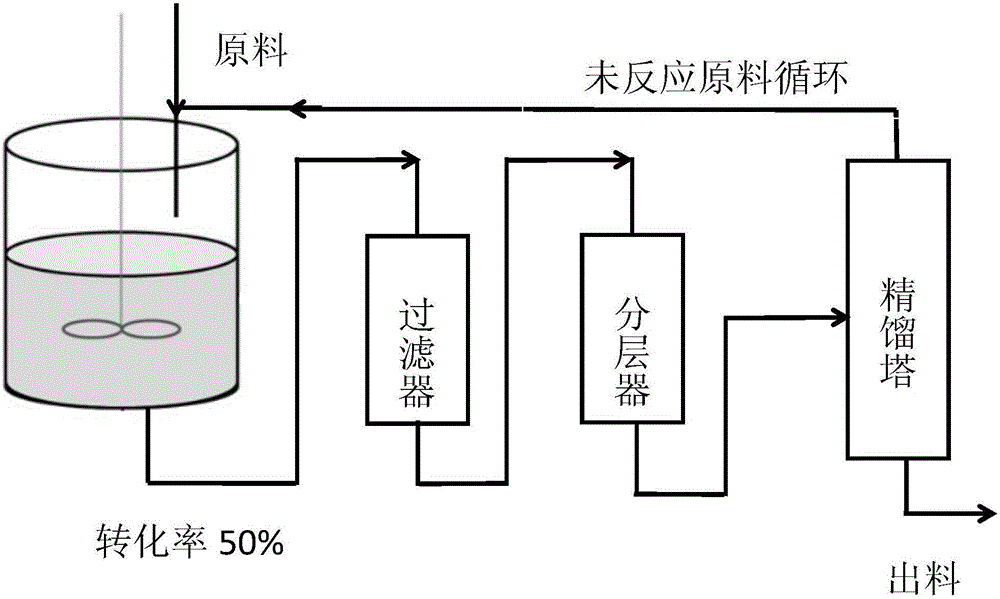
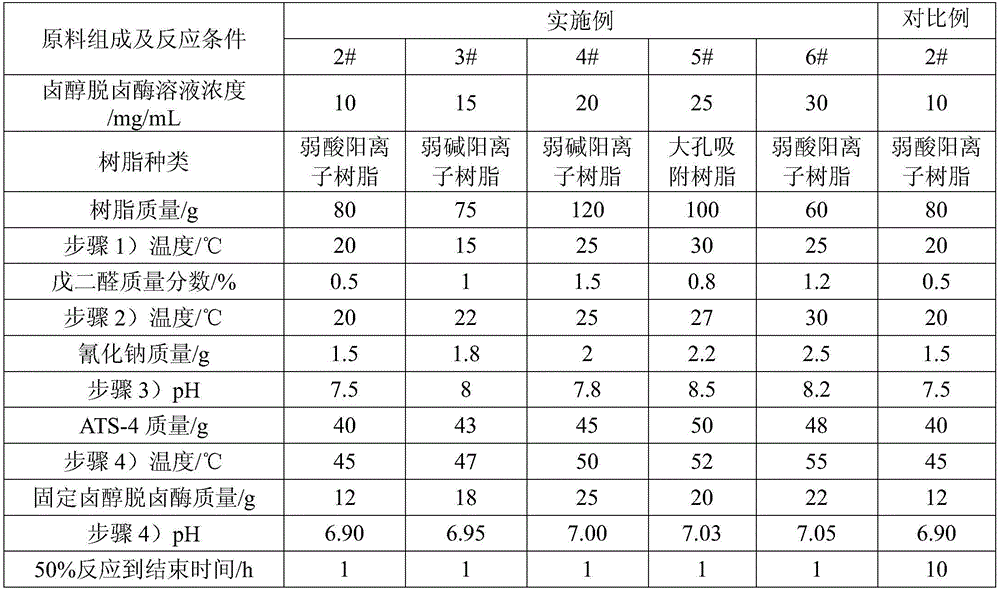
[0029] 1) Prepare a halohydrin dehalogenase solution with a concentration of 20mg / mL, add 40g of adsorption resin to 200mL of halohydrin dehalogenase solution, and stir at 20°C for 3-5h, so that the halohydrin dehalogenase is adsorbed to on the absorbent resin;
[0030] 2) Drop an aqueous glutaraldehyde solution with a mass fraction of 1% into the halohydrin dehalogenase solution in step 1), the volume ratio of the aqueous glutaraldehyde solution to the enzyme solution is 1:25, and at a temperature of 20°C, Stir the reaction for 3-5 hours to obtain a fixative, separate the adsorption resin from the enzyme solution, and wash to obtain the immobilized halohydrin dehalogenase;
[0031] 3) Weigh 210g of distilled water and 1.5g of sodium cyanide, add them to the stainless steel reaction kettle one by one, and adjust the pH to 7.5;
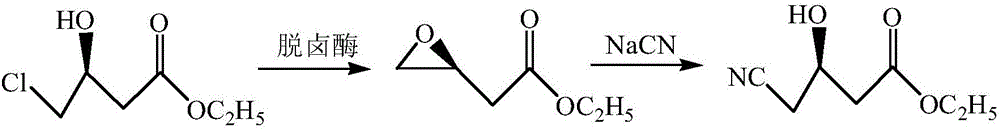
[0032] 4) Add 45g of substrate (S)-4-chloro-3-hydroxybutyric acid ethyl ester (ATS-4), and raise the temperature. When the temperature is 50°C, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com