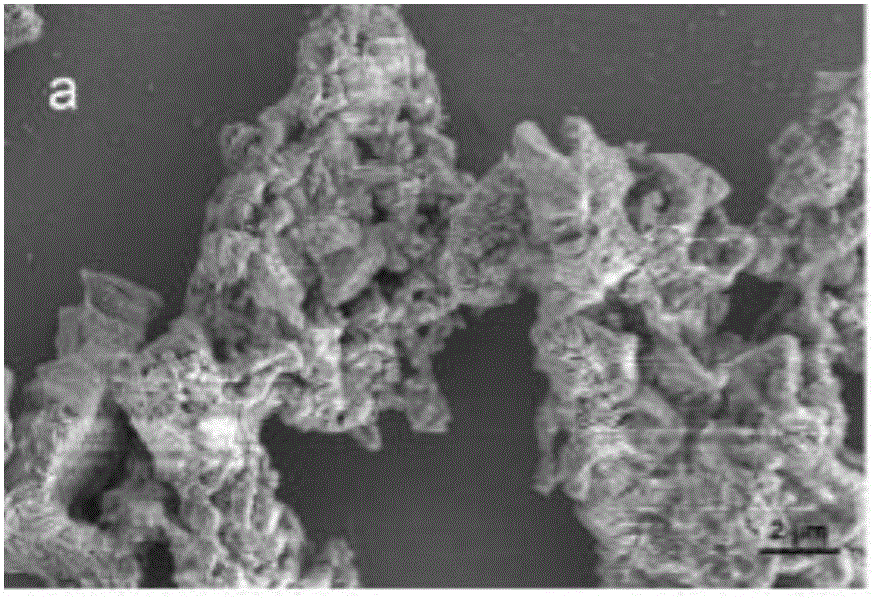
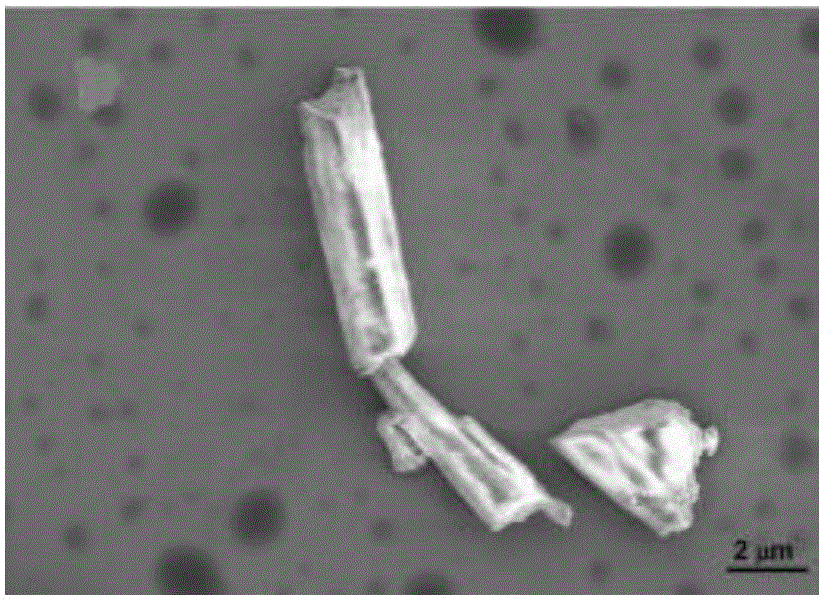
Preparation method of silver-loaded shell powder antibacterial agent
A technology of shell powder and antibacterial agent, which is applied in the field of preparation of silver-loaded shell powder antibacterial agent, can solve problems such as shell pollution, achieve enhanced antibacterial effect, rich resources, and good and sustained antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] Simultaneously, in the preparation process of the shell powder of the present invention, the treatment process for the secondary calcination of the shell powder can also be carried out after the synthesis of the silver-loaded shell powder. That is, the respective reaction processes of the final synthetic product include the following:
[0066] 1) Secondary calcination after shell powder sodium borohydride method combined with silver
[0067] 2) Secondary calcination after the shell powder is combined with silver by ultraviolet irradiation
[0068] 3) Secondary sintered shell powder combined with silver by sodium borohydride method
[0069] 4) Secondary sintered shell powder combined with silver by ultraviolet irradiation
[0070] 5) One-time Ar sintering with sodium borohydride method combined with silver and then calcination.
[0071] Although the synthetic product of the present invention can be finally obtained through the above synthetic routes, the best method o...
Embodiment 1
[0072] Embodiment 1 inhibition zone experiment
[0073] Indicator species: Escherichia coli.
[0074] Sterilization preparation: tweezers, Oxford cup (inner diameter 6mm±0.2mm), petri dish, double distilled water, calcium oxide control (AR Tianjin Bodi Chemical Co., Ltd.).
[0075] Required equipment: biochemical incubator (Hitachi Hitachi Co., Ltd.), ultra-clean bench (Thermo Fisher Scientific).
[0076] Bacteria preparation:
[0077] Dilute Escherichia coli strains frozen at minus 20 degrees Celsius at 1:100 in liquid medium, and activate the strains on a 37°C shaker at 220rpm for 12-14h. After activation, the bacterial solution was stored in a refrigerator at 4°C. Save it for later.
[0078] Sample preparation: Take the same mass of each sample and place it in an EP tube. After high-temperature sterilization, add sterilized double-distilled water dropwise according to the concentration ratio, and perform ultrasonic diffusion treatment to make the solid particles more fi...
Embodiment 2
[0106] Embodiment 2 Minimum Inhibitory Concentration Determination
[0107] From the experimental results of Example 1, the antibacterial sample made by the sodium borohydride reduction method has the strongest antibacterial ability, and this type of sample is selected for further experiments:
[0108] Preparation of bacterial suspension: take 10 μL of Escherichia coli activated in the above experiment, and incubate on a shaker at 37°C until the logarithmic growth phase (about 20 minutes).
[0109] Prepare medium:
[0110] The formula of nutrient broth NB medium is as follows: (100ml)
[0111] Peptone 1.0g (BR Hangzhou Best Biotechnology Co., Ltd.)
[0112] Beef extract 0.3g (BR Shanghai Sangon Bioengineering Co., Ltd.)
[0113] NaCl 0.5g (AR Shanghai Shisi Hewei Chemical Co., Ltd.)
[0114] PH 7.2±0.2(25℃)
[0115] Dilute the sample antibacterial agent: Take 13 sterilized test tubes and add 1.6ml of culture medium to the first tube. Add 1ml of culture medium to each of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com