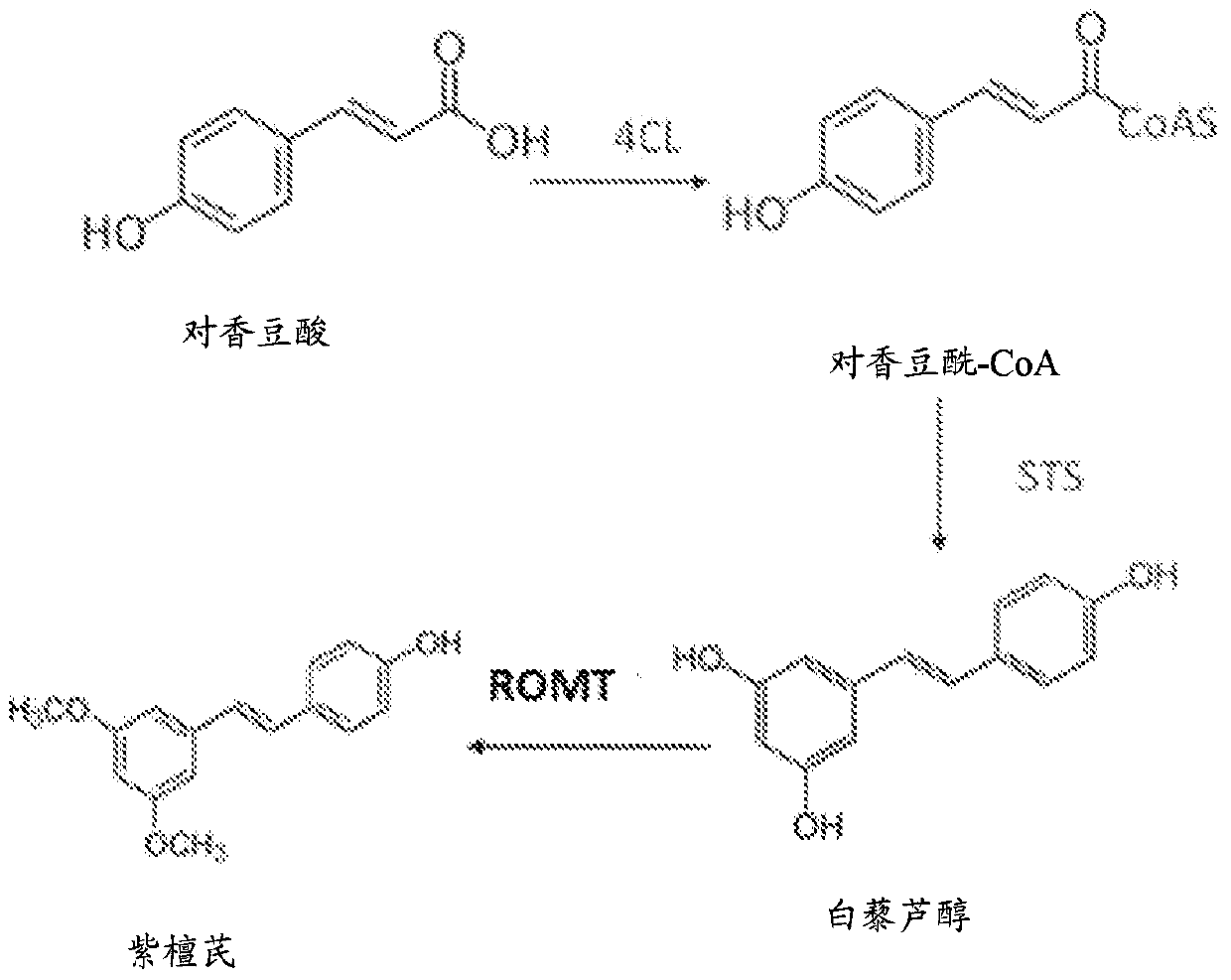
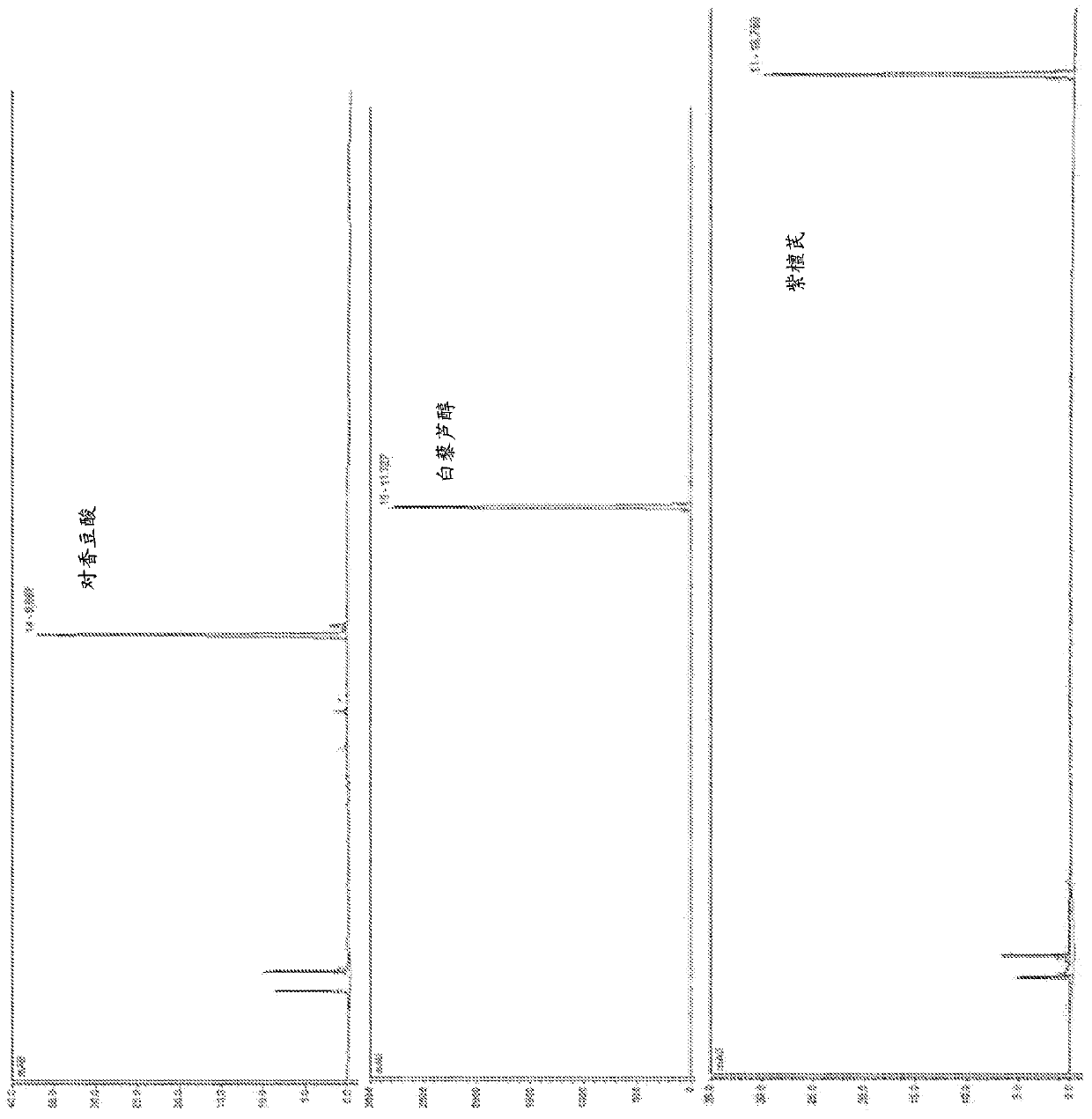
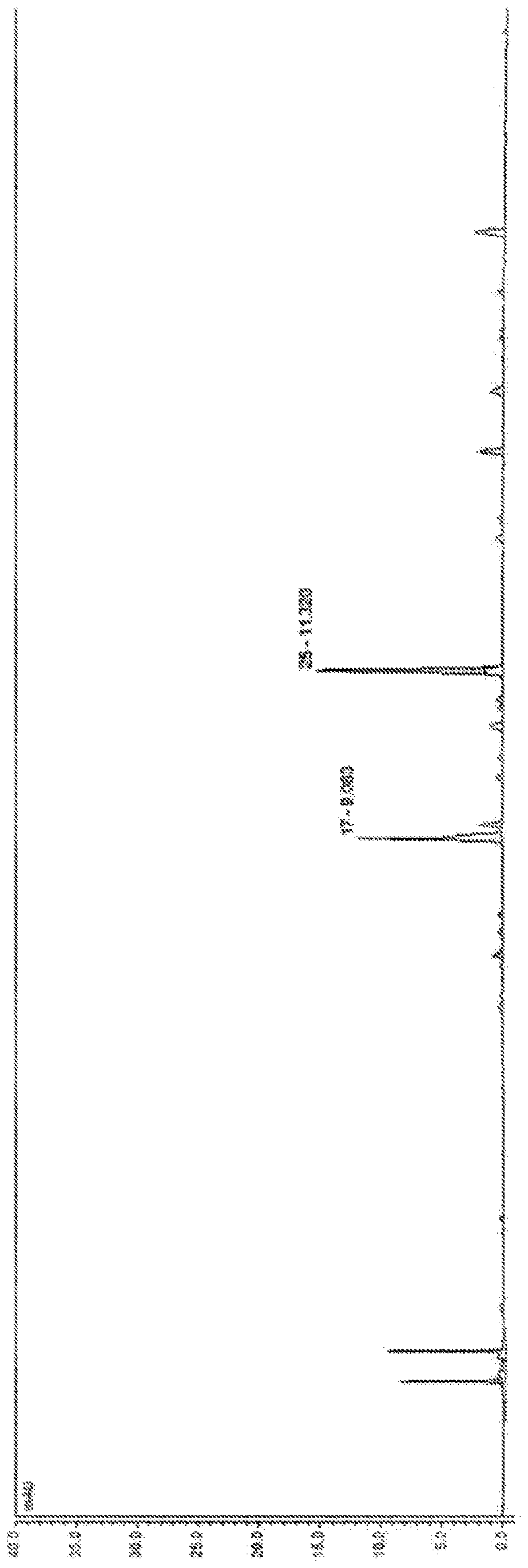
Method for producing pterostilbene biosynthetically using o-methyltransferase
A methyltransferase, biosynthesis technology, used in medicine and pharmacology industry, food field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Construction of bacterial expression vectors
[0054] The PCR product of ROMT was cloned into pETite N-His SUMO Kan vector (Lucigen) according to the manufacturer's manual. The resulting plasmid with the right insert was confirmed by sequencing, Sumo-ROMT, and transformed into BL21(DE3) for heterologous gene expression.
[0055] To construct the 4CL::STS fusion gene, At4CL and VvSTS were fused using a PCR amplification strategy. The stop codon of 4CL was removed and a three amino acid linker (Gly-Ser-Gly) was introduced between the open reading frame of 4CL and the STS. This construction resulted in a 2.87 kb fusion gene construct encoding 4CL, tripeptide linker and STS. The fusion gene 4CL::STS cloned into Gateway entry vector using pCR8 / GW / TOPO TA cloning kit (Invitrogen) was transformed into single-use E. coli cells and then sequenced. The 4CL::STS fusion gene was amplified and cloned via BamHI / HindIII into the multiple cloning site of the pETDuet-1 vector, desi...
Embodiment 2
[0057] Construction of Yeast Expression Vectors
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com