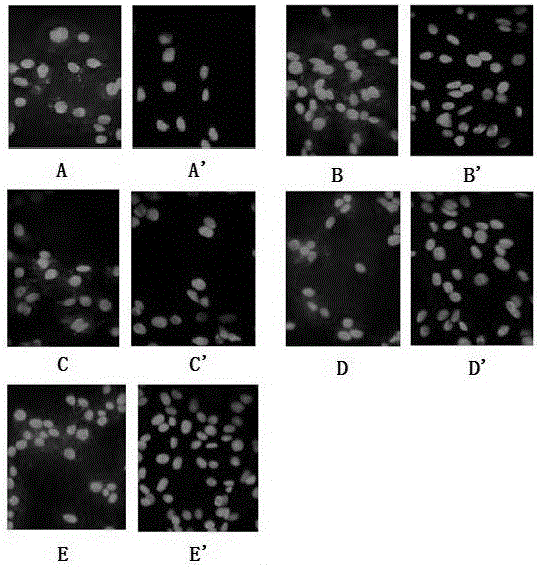
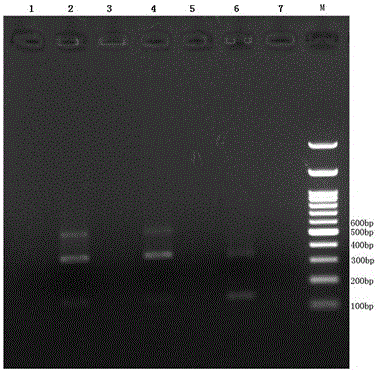
Reagent capable of preventing and eliminating pollution of mammalian cells
A technology of mammals and reagents, which is applied in the field of microbial pollution control in mammalian cell culture, can solve problems such as hindering the synthesis of bacterial cell walls, small antimicrobial peptide molecules, and cell damage, and achieve the removal of bacterial and mycoplasma pollution, normal cell morphology, and prevention pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 A reagent A for preventing and removing contamination of mammalian cells and its preparation method
[0037] Reagent A formula is as follows:
[0038] Peptides: 2.5%
[0039] Lysine: 0.85%
[0040] Methionine: 0.09%
[0041]Sterilize pure water at 121°C and 103Kpa, and refrigerate at 4°C for later use; take 0.25g of polypeptide, 0.085g of lysine, and 0.009g of methionine; add 9ml of pure water into the dissolution bucket in the ultra-clean bench Add polypeptide, lysine and methionine in sequence, stir to dissolve, make up to 10ml with pure water; filter and sterilize the above solution through a 0.22um disposable filter in the ultra-clean bench, sub-package, and store at -20°C Preserve under, obtain reagent A of the present invention.
Embodiment 2
[0042] Example 2 A reagent B for preventing and removing contamination of mammalian cells and its preparation method
[0043] Reagent B formula is as follows:
[0044] Peptides: 3.8%
[0045] Lysine: 1.56%
[0046] Methionine: 0.36%
[0047] Sterilize the PBS solution at a high temperature of 121°C and 103Kpa, and refrigerate it at 4°C for later use; take 0.38g of polypeptide, 0.156g of lysine, and 0.036g of methionine; add 9ml of PBS solution into the dissolution bucket in the ultra-clean bench , add polypeptide, lysine and methionine in turn, stir to dissolve, make up to 10ml with PBS solution; filter and sterilize the above solution through a 0.22um disposable filter in the ultra-clean bench, sub-package, and store at -20°C Preserve, obtain reagent B of the present invention.
Embodiment 3
[0048] Example 3 A reagent C for preventing and removing contamination of mammalian cells and its preparation method
[0049] Reagent C formula is as follows:
[0050] Peptides: 7.3%
[0051] Lysine: 1.82%
[0052] Methionine: 0.58%
[0053] Sterilize the DMEM liquid medium at 121°C and 103Kpa, and refrigerate it at 4°C for later use; take 0.73g of polypeptide, 0.182g of lysine, and 0.058g of methionine; add 9ml of DMEM solution in the ultra-clean bench to dissolve In the bucket, add polypeptide, lysine and methionine in sequence, stir to dissolve, make up to 10ml with DMEM; filter and sterilize the above solution through a 0.22um disposable filter in the ultra-clean bench, subpackage, and store at -20°C Preserve under, obtain reagent C of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com