2-(2'-hydroxyl styryl) naphthyridine probe reagent and preparation and application thereof
A technology of hydroxystyrene and methylnaphthyridine, used in chemical instruments and methods, analysis by chemical reaction of materials, material analysis by observing the effect on chemical indicators, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] 2-(2'-hydroxystyryl) naphthyridine probe reagent synthesis, specifically comprises the following steps
[0111] a. Synthesis of intermediate 2-methylnaphthyridine:
[0112] Under the condition of ice bath, add concentrated H 2 SO 4 41g (22mL), under stirring, add 17.5g (78mmol) of sodium m-nitrobenzenesulfonate, 2.4g (39mmol) of boric acid, FeSO 4 ·7H 2 O 1.4g (5mmol), glycerol 12.5mL, 2-amino-6-picoline 4.3g (40mmol), mix well, then add 22.5mL of warm water at 50°C, reflux at 135°C for 2-3h, cool After that, adjust to alkaline with 50% NaOH solution, filter with suction, extract the aqueous solution with chloroform three times, and use anhydrous MgSO as the organic layer 4 Drying, rotary evaporation to remove the solvent to obtain a crude product, recrystallization of the crude product with cyclohexane to obtain 2-methylnaphthyridine, namely the probe reagent; the concentration of the concentrated sulfuric acid is 95-98%;
[0113] b. Synthesis of 2-(2'-hydroxystyr...
Embodiment 2
[0116] The preparation method of various reagents in the analytical method of the present invention:
[0117] (1) Preparation of probe stock solution: Weigh 25 mg of the probe reagent prepared in Example 1, dissolve N,N-dimethylformamide and prepare N,N-dimethylformamide with a concentration of 0.1 mM Probe stock solution 10mL;
[0118] Weigh 25 mg of the probe reagent prepared in Example 1, dissolve 1,4-dioxane and prepare 10 mL of 1,4-dioxane probe stock solution with a concentration of 0.1 mM.
[0119] Weigh 25 mg of the probe reagent prepared in Example 1, dissolve in acetonitrile and prepare 10 mL of acetonitrile probe stock solution with a concentration of 0.1 mM.
[0120] (2)Hg 2+ Preparation of stock solution: Weigh 90.4 mg of mercury perchlorate tetrahydrate, dissolve it in water and prepare 10 mL of a solution with a concentration of 20 mM.
[0121] (3)Ag + Preparation of stock solution: Weigh 41.5 mg of silver perchlorate monohydrate, dissolve it in water and pr...
Embodiment 3
[0128] Embodiment 3: fluorescence spectrometry is to Hg 2+ 、Ag + , F - detection
[0129] 1. Detection of Hg 2+ :
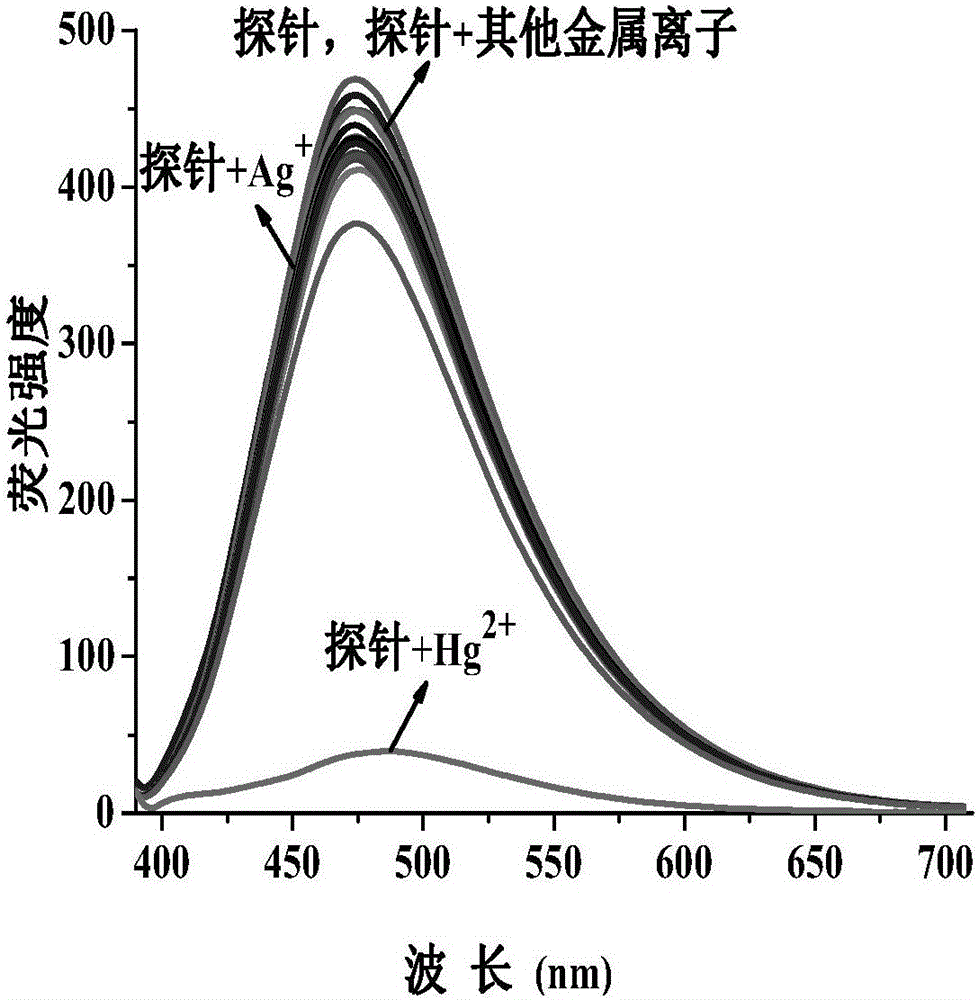
[0130] Set the fluorescence excitation wavelength to 375nm, add N,N-dimethylformamide probe stock solution (0.1mM, 1mL) into a 10mL volumetric flask, and use N,N-dimethylformamide / H 2 Dilute O to the mark to make N,N-dimethylformamide / H 2 O is 19 / 1 (v / v), shake well to make a probe solution, take 3mL of the solution in a 1cm cuvette for fluorescence spectrum measurement. After adding N,N-dimethylformamide probe stock solution (0.1mM, 1mL) to a series of 10mL volumetric flasks, add metal ion Hg 2+ , Ag + , Li + , Na + , K + , Mg 2+ , Ca 2+ , Ba 2+ ,Sr 2+ , Zn 2+ , Al 3+ ,Co 2+ , Ni 2+ , Cu 2+ , Zn 2+ , Pb 2+ , Cd 2+ , La 3+ Stock solution (20mM, 0.1mL);
[0131] In N,N-dimethylformamide / H 2 In O(v / v, 19 / 1) solution, the probe solution with a concentration of 10μM emits a strong blue fluorescence at 475nm under excitation at a wavelength of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com