PET developing agent precursor TsOP-(+)-DTBZ and separation and measurement method of optical isomer thereof
A PET imaging agent and measurement method technology, applied in the field of analytical chemistry, to achieve the effect of good peak symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Instruments and Conditions
[0046] Mobile phase: n-hexane-1,2-dichloroethane-absolute ethanol-trifluoroacetic acid-triethylamine=65:33:2:0.01:0.005
[0047] Flow rate: 0.8mL / min
[0048] Column temperature: 25°C
[0049] Injection volume: 20μL
[0050] UV detection wavelength 280nm
[0051] Experimental steps:
[0052] Accurately weigh 5 mg of TsOP-(+)-DTBZ and its isomers respectively, put them in a 10 mL brown volumetric flask, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution. Get need testing solution and carry out high performance liquid chromatography analysis according to above-mentioned conditions, record chromatogram.
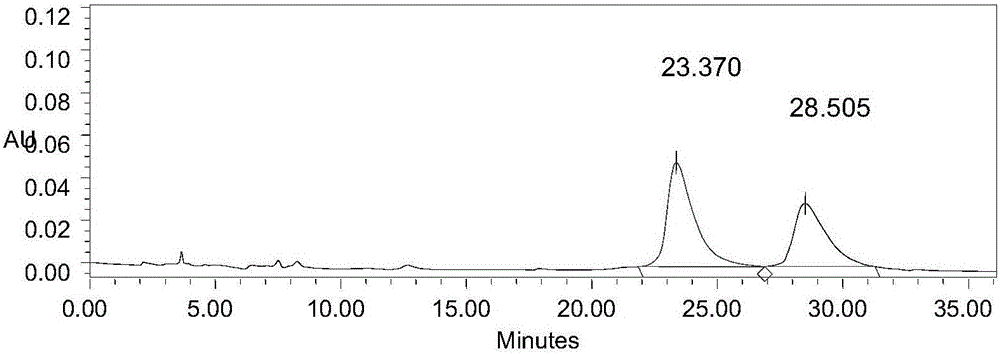
[0053] see attached results figure 1 , it can be seen that TsOP-(+)-DTBZ and its isomers achieve baseline separation under this condition, and the high performance liquid chromatography shows a resolution of 2.5.
Embodiment 2
[0055] Instruments and Conditions
[0056] Mobile phase: n-hexane-1,2-dichloroethane-absolute ethanol-trifluoroacetic acid-triethylamine=64.6:32.3:3.1:0.01:0.005
[0057] Flow rate: 0.8mL / min
[0058] Column temperature: 30°C
[0059] Injection volume: 20μL
[0060] UV detection wavelength 280nm
[0061] Experimental steps:
[0062] Accurately weigh 10 mg of TsOP-(+)-DTBZ and its isomers respectively, put them in a 10 mL brown volumetric flask, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution. Get need testing solution and carry out high performance liquid chromatography analysis according to above-mentioned conditions, record chromatogram.
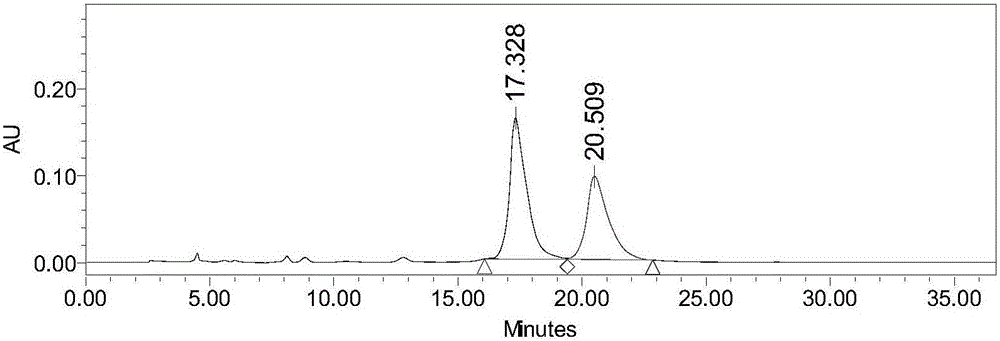
[0063] see attached results figure 2 , showing that the separation and detection method of the present invention can separate TsOP-(+)-DTBZ and its isomers, and the high-performance liquid chromatography shows that the separation degree is 2.2.
Embodiment 3
[0065] Instruments and Conditions
[0066] Mobile phase: n-hexane-1,2-dichloroethane-absolute ethanol-trifluoroacetic acid-triethylamine=64:32:4:0.01:0.005
[0067] Flow rate: 0.8mL / min
[0068] Column temperature: 25°C
[0069] Injection volume: 20μL
[0070] UV detection wavelength 280nm
[0071] Experimental steps:
[0072] Accurately weigh 5 mg of TsOP-(+)-DTBZ and its isomers respectively, put them in a 10 mL brown volumetric flask, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution 1. In addition, accurately weigh 10 mg of TsOP-(+)-DTBZ bulk drug, put it in a 10 mL brown volumetric flask, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution 2.
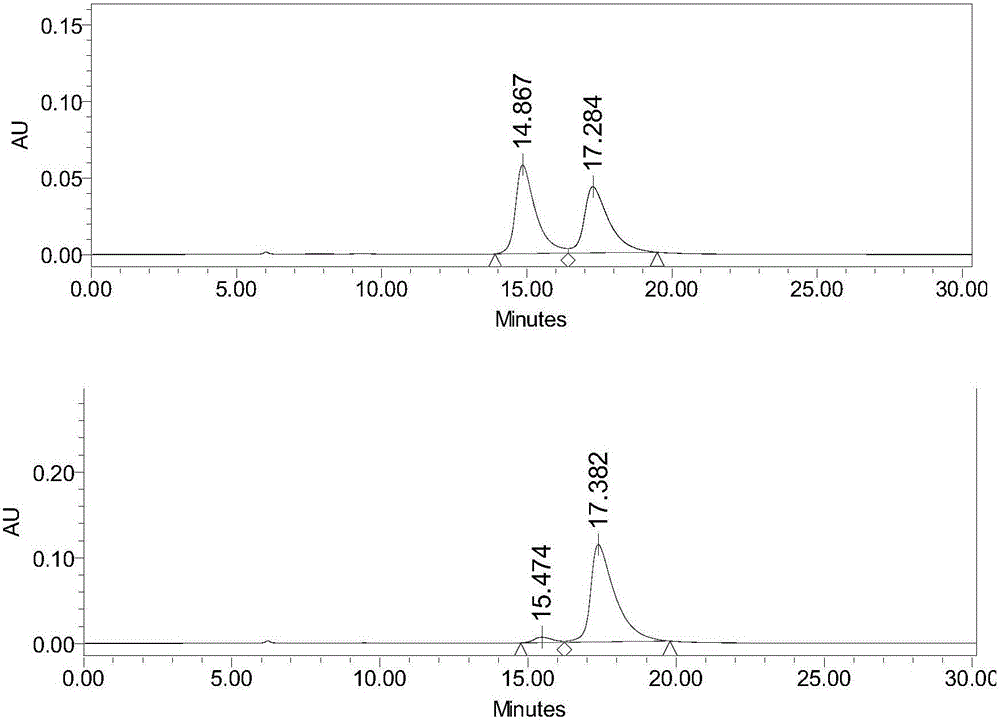
[0073] Get need testing solution and carry out high performance liquid chromatography analysis by above-mentioned conditions, record chromatogram, the result sees attached image 3 .
[0074] image 3 It is proved that this method ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com