New method for preparing (R)-1,2-di-fatty acid glycerol phosphatidyl glyceride
A fatty acid glycerol phosphatidylglycerol, a new method technology, applied in the field of medicine and chemical industry, can solve the problems of using heavy metals or highly toxic oxidants, not suitable for industrial production, complex routes, etc., achieve good optical stability, easy introduction, mild reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
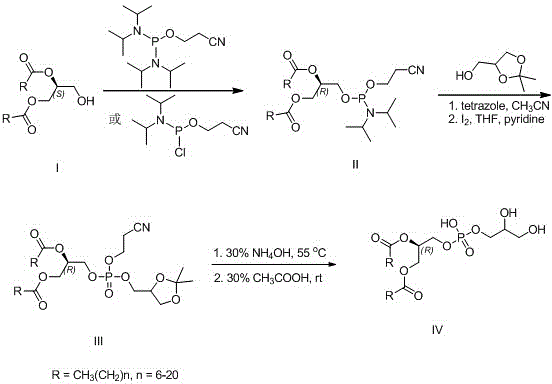
[0026] Preparation of (R)-1,2-distearoyl-[(2-cyanoethoxy)-(diisopropylamino)-phosphooxy]glyceride (R=CH 3 (CH 2 ) 16 )(method one)
[0027] Under argon protection, (S)-1,2-glyceryl stearate (0.62 g, 1 mmol) was dissolved in 10 mL of anhydrous dichloromethane, and 1 mL of diisopropylethylamine was added. At 0°C, 2-cyanoethyldiisopropylphosphoramidate chloride (0.43 mL, 2 mmol) was added dropwise, and the mixture was allowed to react overnight at room temperature. Add 10 mL of saturated aqueous sodium bicarbonate solution to terminate the reaction, extract with dichloromethane, dry the organic layer over anhydrous sodium sulfate, concentrate, and recrystallize the crude product from ethyl acetate-petroleum ether to obtain 0.62 g of (R)-1,2-dihard Fatty acyl-[(2-cyanoethoxy)-(diisopropylamino)-phosphooxy]glycerides, yield 75%.
Embodiment 2
[0029] Preparation of (R)-1,2-distearoyl-[(2-cyanoethoxy)-(diisopropylamino)-phosphooxy]glyceride (R=CH 3 (CH 2 ) 16 )(Method Two)
[0030] Under argon protection, (S)-1,2-glyceryl stearate (0.62g, 1 mmol) was dissolved in 10 mL of anhydrous dichloromethane, and 5-ethylthiotetrazolium (0.13g, 1 mmol), bis(diisopropylamino)(2-cyanoethoxy)phosphine (0.24 g, 1 mmol) was added dropwise, and heated to reflux to react overnight. Add 10 mL of saturated aqueous sodium bicarbonate solution to terminate the reaction, extract with dichloromethane, dry the organic layer over anhydrous sodium sulfate, concentrate and recrystallize the crude product from ethyl acetate-petroleum ether to obtain 0.57 g of (R)-1,2-distearyl Acyl-[(2-cyanoethoxy)-(diisopropylamino)-phosphoryloxy]glycerides, yield 69%.
Embodiment 3
[0032] (R)-1,2-Distearoyl-[(2-cyanoethoxy)-(2,2-dimethyl-1,3-dioxo-4-yl)methoxy-]phospholipid Preparation of acylglycerides (R = CH 3 (CH 2 ) 16 )
[0033] (R)-1,2-distearoyl-[(2-cyanoethoxy)-(diisopropylamino)-phosphoryloxy]glyceride (0.82 g, 1 mmol) in 10 mL of anhydrous acetonitrile, Then 15 mL of anhydrous acetonitrile solution of 2,2-dimethyl-4-hydroxymethyl-1,3-dioxolane (180 μL, 1.5 mmol) and 2.5% tetrazole (5 mL) was added dropwise into the above solution and stirred at room temperature for 1 hour. Concentrate to dryness, add 0.1 M iodine tetrahydrofuran-water-pyridine (9:1:0.1) mixed solution to the concentrate, stir and react for 1 hour, then concentrate to dryness, add chloroform, then use sodium sulfite The aqueous solution and saturated brine were washed, the organic layer was anhydrous sodium sulfate, concentrated and directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com