Preparation method of chloroprocaine hydrochloride
A technology of chloroprocaine hydrochloride and o-chlorine, which is applied in the field of organic drug synthesis, can solve the problems of low production efficiency, long reaction period, reduced reaction energy consumption, etc., and achieves reduced production period, short reaction time, and improved reaction yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
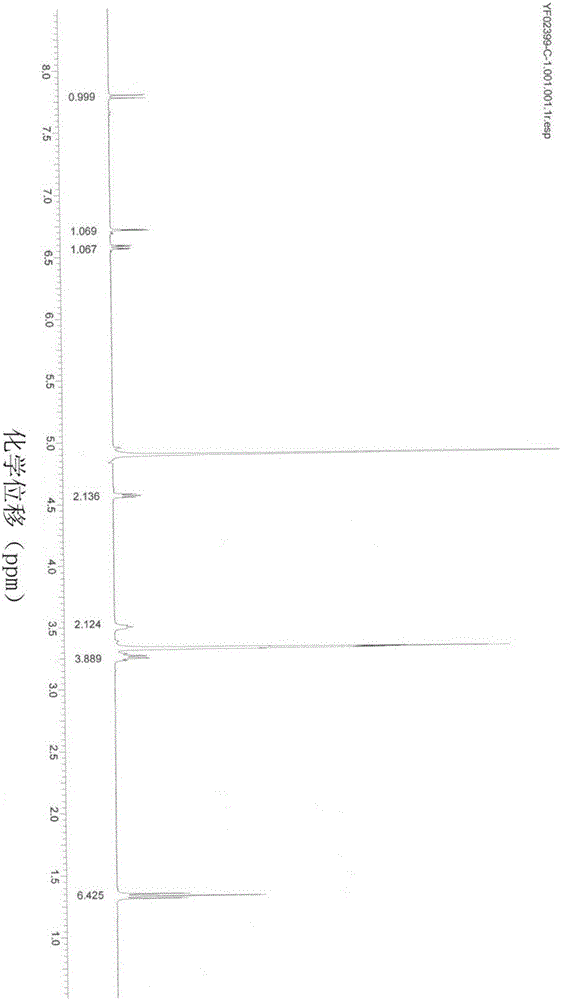
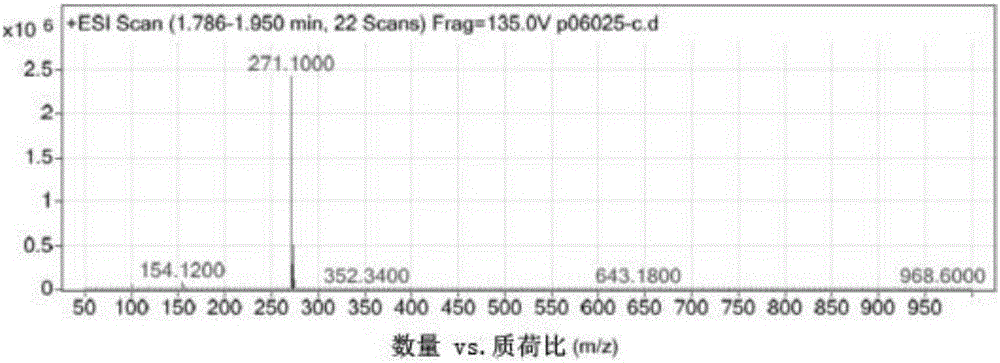
Image
Examples
Embodiment 1
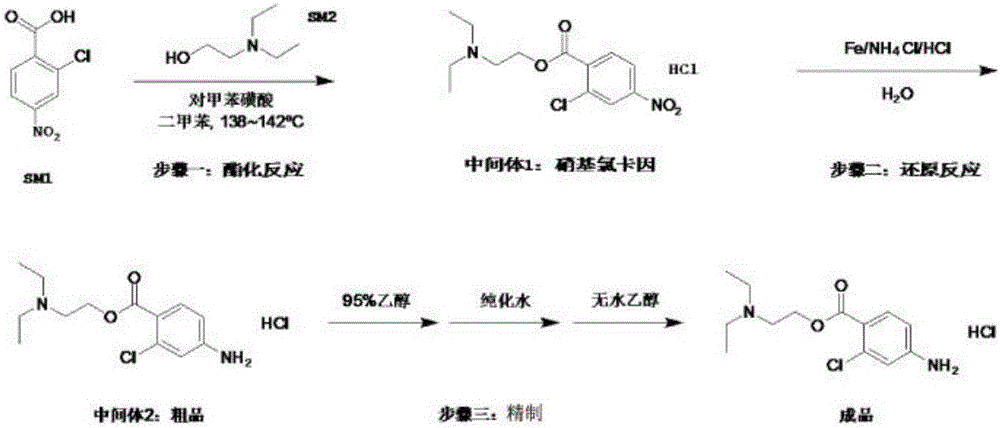
[0034] Such as image 3 As shown, step 1: under stirring at room temperature, dissolve 1 mol of o-chloro-p-nitrobenzoic acid and 3 mol of 2-diethylaminoethanol in xylene until the solution is clear, and then add p-toluenesulfonic acid. The reactant was heated to 138-142°C, refluxed, and reacted for 2 hours. After the reaction was completed, it was cooled to room temperature and filtered. The filtrates were washed 3 times with aqueous NaOH respectively. The organic phase was extracted twice with hydrochloric acid solution, and the combined extracts were adjusted to pH 3.4-4.0 to obtain an aqueous solution of intermediate 1 (nitrolocaine), which was set aside.
[0035] Step 2: Add the aqueous solution of intermediate 1 into the reaction flask, add ammonium chloride, and add reducing iron powder in batches. Heat to 68-70°C, stir for 2-3 hours. After the reaction is completed, filter while it is hot. After the filtrate is acidified, add a saturated solution of sodium sulfide d...
Embodiment 2
[0044] Step 1: Dissolve 1 mol of o-chloro-p-nitrobenzoic acid and 3 mol of 2-diethylaminoethanol in xylene under stirring at room temperature, and then add p-toluenesulfonic acid until the solution is clear. The reactant was heated to 138-142°C, refluxed, and reacted for 4 hours. After the reaction was completed, it was cooled to room temperature and filtered. The filtrates were washed 3 times with aqueous NaOH respectively. The organic phase was extracted twice with hydrochloric acid solution, and the combined extracts were adjusted to pH 3.4-4.0 to obtain an aqueous solution of intermediate 1 (nitrolocaine), which was set aside.
[0045] Step 2: Add the aqueous solution of intermediate 1 into the reaction flask, add ammonium chloride, and add reducing iron powder in batches. Heated to 80°C and stirred for 1h. After the reaction is completed, filter while it is hot. After the filtrate is acidified, add a saturated solution of sodium sulfide dropwise to make it alkaline and...
Embodiment 3
[0054] Step 1: Dissolve 1mol o-chloro-p-nitrobenzoic acid and 3mol 2-diethylaminoethanol in xylene under stirring at room temperature until the solution is clear, then add immobilized liquid acid HF / Al 2 O. The reactant was heated to 138-142°C, refluxed, and reacted for 6 hours. After the reaction was completed, it was cooled to room temperature and filtered. The filtrates were washed 3 times with aqueous NaOH respectively. The organic phase was extracted twice with hydrochloric acid solution, and the combined extracts were adjusted to pH 3.4-4.0 to obtain an aqueous solution of intermediate 1 (nitrolocaine), which was set aside.
[0055] Step 2: Add the aqueous solution of intermediate 1 into the reaction flask, add ammonium chloride, and add reducing iron powder in batches. 25 ° C, stirring the reaction for 4h. After the reaction is finished, filter, and the filtrate is acidified, and a saturated solution of sodium sulfide is added dropwise to make it alkaline, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com