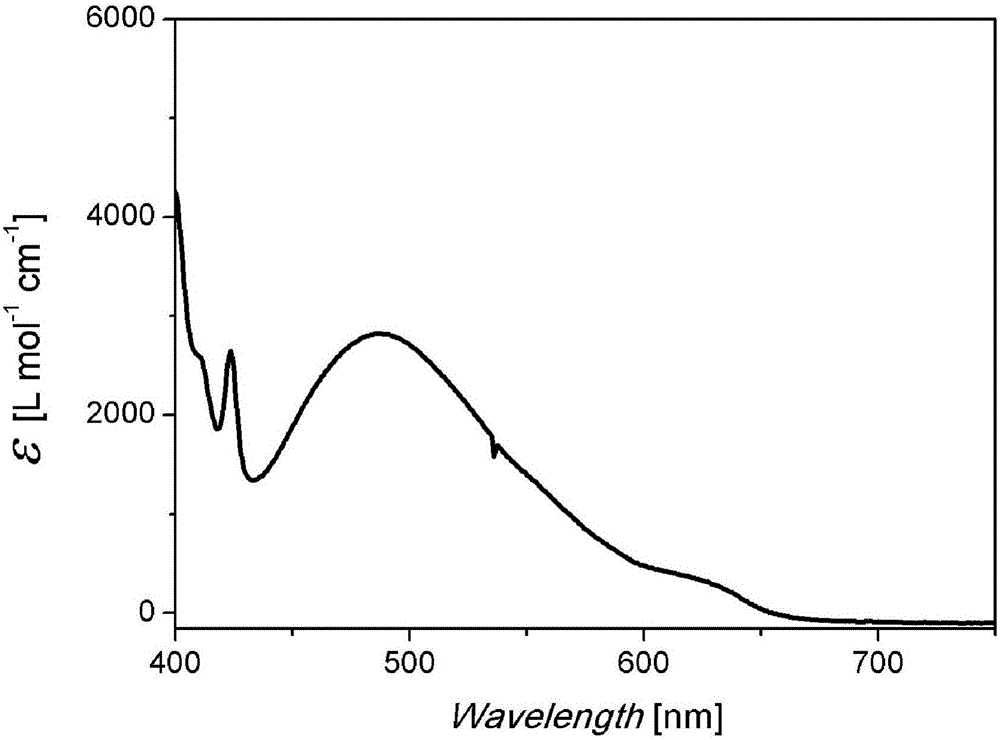
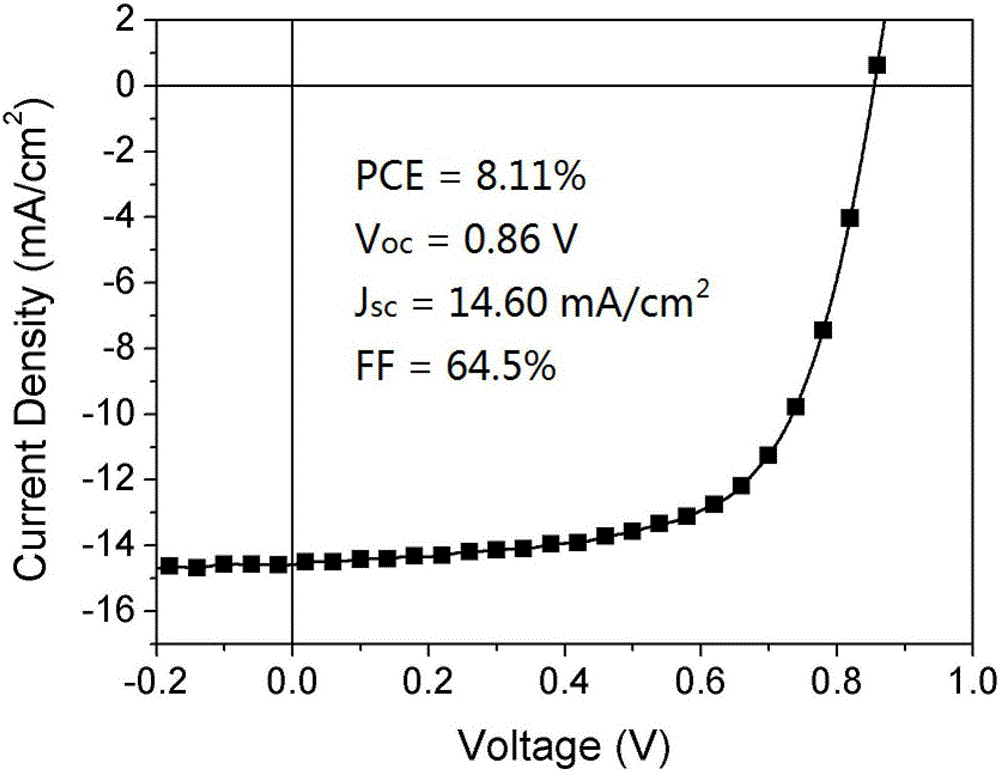
Pure heterogeneous fullerene bis-addition derivative and preparation method thereof
A fullerene and double-addition technology, which is applied in the field of fullerene double-addition derivatives and its preparation, can solve the problems of increasing trap state density, lack of regioselectivity, and reduced fullerene electron mobility. Achieve high Voc sum, good solubility and high energy conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: Preparation of carbon sixty-two anthracene addition products
[0066] Mix 13.9mmol of C60 and 27.8mmol of anthracene solid powder in an agate mortar, then transfer to a 25mL round-bottom single-mouth bottle, equip the single-mouth bottle with a glass vacuum valve, vacuum the system for 5 minutes, and then evacuate it Put it in a muffle furnace and react at 240°C for 2 hours. After cooling, take out the round bottom bottle and open the vacuum valve to balance the air pressure inside and outside the bottle. Dig out the reacted solid with a spoon and wash it twice with carbon disulfide (200mL) to obtain C6 The decabisanthracene addition product, which contains unreacted C60 and C60bisanthracene addition product at a ratio of about 1:1, 12.6g, can be directly used as a raw material for the next step reaction.
[0067] 1 H NMR (CDCl 3 / CS 2 , 400MHz, δ / ppm): 7.81-7.83 (m, 8H, Ar), 7.49-7.51 (m, 8H, Ar), 6.07 (s, 4H, bridgehead CH).
Embodiment 2
[0068] Embodiment 2: the Bingel single addition product of preparation carbon sixty-two anthracene addition products
[0069] The synthesis process is as follows:
[0070]
[0071] 12.6 g of the C60 bis-anthracene addition product prepared in Example 1 were dispersed in carbon disulfide (1200 mL), and 56 mmol of di-tert-butyl bromomalonate and 56 mmol of DBU were added successively, and reacted at room temperature for 16 hours. After the end, the solid was filtered off, and the filtrate was directly washed with a gradient on a silica gel column. The developer was first selected carbon disulfide, and passed through the next brown color band. The developing agent is carbon disulfide: dichloromethane=3:1 (v / v), and the next brown-green color band passes through, and the Bingel mono-addition product (3.0 g) is obtained after the solvent is spin-dried. The molecular ion peak of product mass spectrum is 1291.2843, and (M+H + )match; 1 H NMR (CDCl 3 / CS 2 ,400MHz,δ / ppm):7.74-...
Embodiment 3
[0072] Embodiment 3: prepare the Bingel double addition product of carbon sixty-two anthracene addition product
[0073] The synthesis process is as follows:
[0074]
[0075] 2.3 mmol of the Bingel mono-addition product prepared in Example 2 were dispersed in o-dichlorobenzene (300 mL), and 6.9 mmol of di-tert-butyl bromomalonate and 6.9 mmol of potassium tert-butoxide were added successively, at room temperature Reacted for 15 minutes, quenched with water, and the organic phase was directly washed with a gradient of silica gel column. The developing agent was first selected carbon disulfide, followed by o-dichlorobenzene, and then the developing agent was selected carbon disulfide: dichloromethane=2:1 (v / v), The first brown color band is the unreacted raw material, and the second red color band is the Bingel double addition product, and 1.5 g is obtained after spin-drying the solvent. The molecular ion peak of product mass spectrum is 1505.4021, and (M+H + )match; 1 H ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com