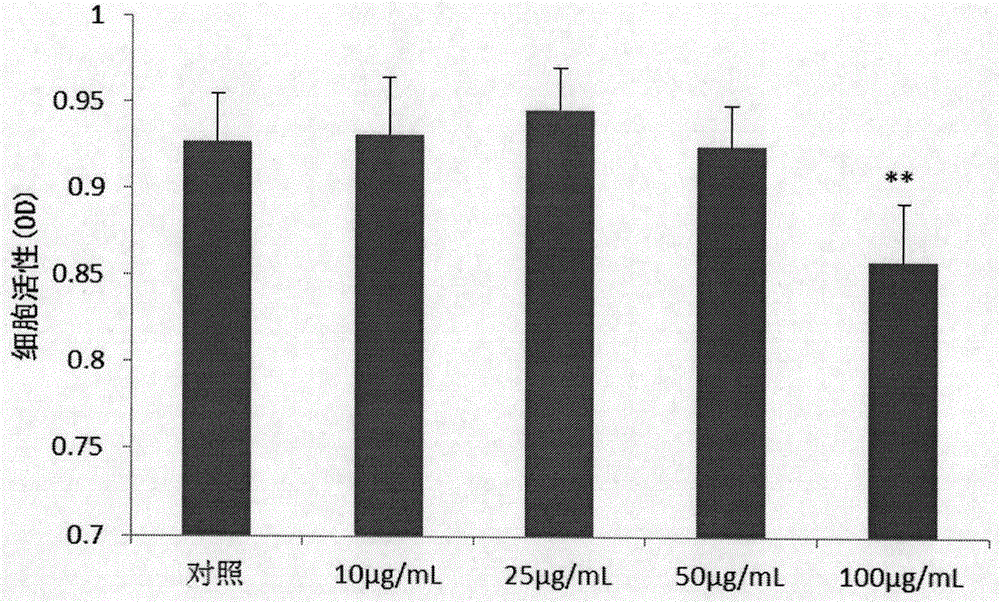
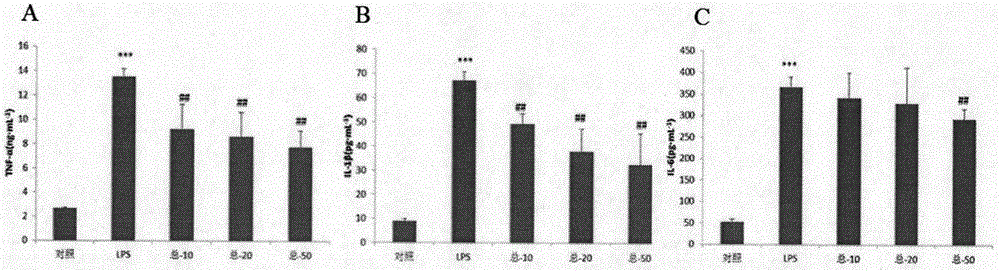
Application of sanguisorba total saponin to treatment of inflammatory bowel diseases
A technology of inflammatory bowel disease and Burnet saponins, which is applied in the field of medicine, can solve the problems of loss of normal flora tolerance and reduced immune response resistance of intestinal bacteria.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Qualitative Analysis of Saponins Components in Example 1 Burnet Extract
[0017] Step 1: Analysis of the fragmentation mode of Burnet saponins: Saponins will undergo deglycosylation reaction, 162Da means removal of hexose (glucose, Glc-), 132Da means removal of pentose (arabinose, Ara-). Analysis of the fragment information found that in the secondary fragment mass spectrograms of saponins I and II, 453.33, 471.34 and 585.38 had relatively high intensities. Therefore, we selected the characteristic fragment ions m / z 453.33, 471.34, 585.38 and neutral loss m / z 132, 162 as the characteristic information of saponins, and established the characteristic fragment ion diagnostic technology and neutral loss suitable for the rapid identification of Saponins from Burnet eucalyptus. technology.
[0018] Step 2: Through the characteristic ion diagnosis technology and neutral loss technology, 24 saponin components were comprehensively and rapidly identified for the first time. The ...
Embodiment 2
[0022] Example 2 Identification of In vitro Metabolites of Burnet Saponins in Normal and Inflammatory Bowel Disease Model Rats
[0023] Step 1: Preparation of TNBS enema enteritis model: TNBS was dissolved in absolute ethanol solution (1:1) to prepare a solution with a concentration of 100 mg / kg. Rats were anesthetized by intraperitoneal injection of 7% chloral hydrate. Draw up the prepared TNBS solution with a syringe, fully lubricate the end of the catheter with Vaseline, and enema, the end of the catheter is about 5 cm away from the anus of the rat, slowly push the TNBS in the syringe into the colon, and invert the rat for 1 min. The mice in the control group were given the same dose of normal saline.
[0024] Step 2: Preparation of intestinal flora: Rats were sacrificed by bleeding from the femoral artery, the ileocecal valve was taken out, its contents were exposed, and the contents of the intestinal tract were weighed. Add 3mL of sample preservation solution (20% glyce...
Embodiment 3
[0031] Example 3 Study on Metabolism of Burnet Saponins in Normal and Inflammatory Bowel Disease Model Rats
[0032] Step 1: Experimental animals and grouping: 24 healthy male SD rats were divided into normal group and model group. The model group was given TNBS (100 mg / kg) anally for 7 days, and the two groups were given Burnet saponin (50 mg / kg) by intragastric administration.
[0033]Step 2: collection of biological samples: blank plasma samples were collected before administration, and sacrificed by femoral artery bloodletting 1 hour after intragastric administration, and the plasma of the model group and the control group were respectively collected in tubes added with sodium heparin. After intragastric administration, they were put into metabolic cages, and urine and feces were collected before administration and at 0-24h, 24-48h, 48-72h, and 72-96h after administration.
[0034] Step 3: Biological sample processing: Take 200 μl of plasma from rats in the control group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com