Aryl benzofuran amidated derivatives and pharmaceutical use thereof
A technology for furan-based amides and arylbenzenes, which is used in the field of arylbenzofuran-based amidated derivatives and their preparation, and can solve problems such as uncertainty, uncertainty, and unclear structure-activity relationship.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
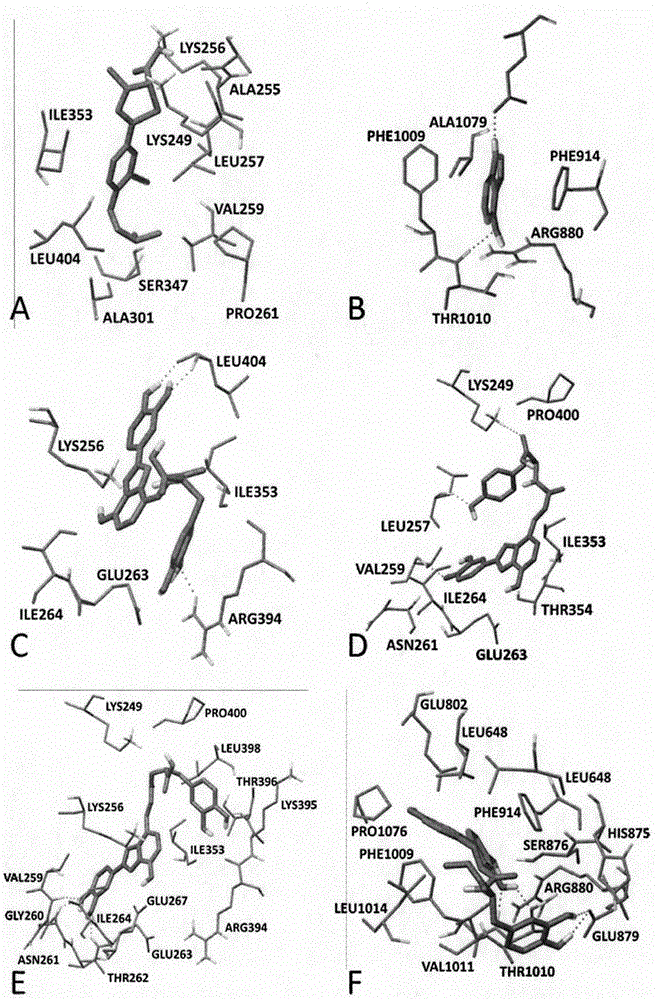
Image
Examples
Embodiment 1
[0132] 1. Synthesis of arylbenzofuran amidated derivatives 5a, 6a
[0133] (1)Tournefolic acid A
[0134] Accurately weigh Salvianolic acid C (1) (245mg, 0.498mmol) in a two-neck round bottom bottle, add MeOH / H 2 O 20mL (5:1v / v), sonicate until the sample is completely dissolved, add an appropriate amount of inorganic base (NaOH or KOH) accurately weighed, and continue TLC under heating conditions (chloroform / methanol / formic acid 8:1:1v / v / v ) to monitor the reaction until the complete disappearance of the starting material. After the reaction is complete, place it at room temperature, evaporate the solvent MeOH to dryness, add 15 mL of distilled water to dilute, and add 10% HCl aqueous solution drop by drop under ice bath cooling with constant stirring until the pH is 3-4, then stop the dropwise addition. Add ethyl acetate (20mL×3) for extraction, combine the organic layers, wash with saturated NaCl solution, MgSO 4 Dry and concentrate under reduced pressure to obtain a cru...
Embodiment 12
[0288] The antioxidant activity of the synthesized benzofuran amidated derivatives was evaluated by DPPH free radical scavenging experiment.
[0289] 12.1 Preparation of reagents and standard solutions
[0290] (1) Preparation of DPPH solution: Accurately weigh an appropriate amount of DPPH, add MeOH for ultrasonic dissolution, prepare a 10 mM stock solution, and store at -20°C. Dilute to 0.1mM with MeOH before the experiment, and store in the dark;
[0291](2) Preparation of the test drug: Accurately weigh an appropriate amount of the test drug, dissolve it in MeOH to prepare a 10m stock solution, and store it in the dark at -20°C. Diluted to different concentrations (0-100mM) with MeOH before the experiment.
[0292] 12.2 Experimental steps
[0293] (1) Add 100 μL of different concentrations of sample solutions to be tested on a 96-well plate, then add 100 μL of 0.1 mM DPPH solution, and use the same volume of MeOH as a blank control and Quercetin as a positive control. ...
Embodiment 13
[0302] The cell model was used to evaluate the antioxidant activity (the ability to scavenge superoxide anion) of the synthesized benzofuran amidated derivatives.
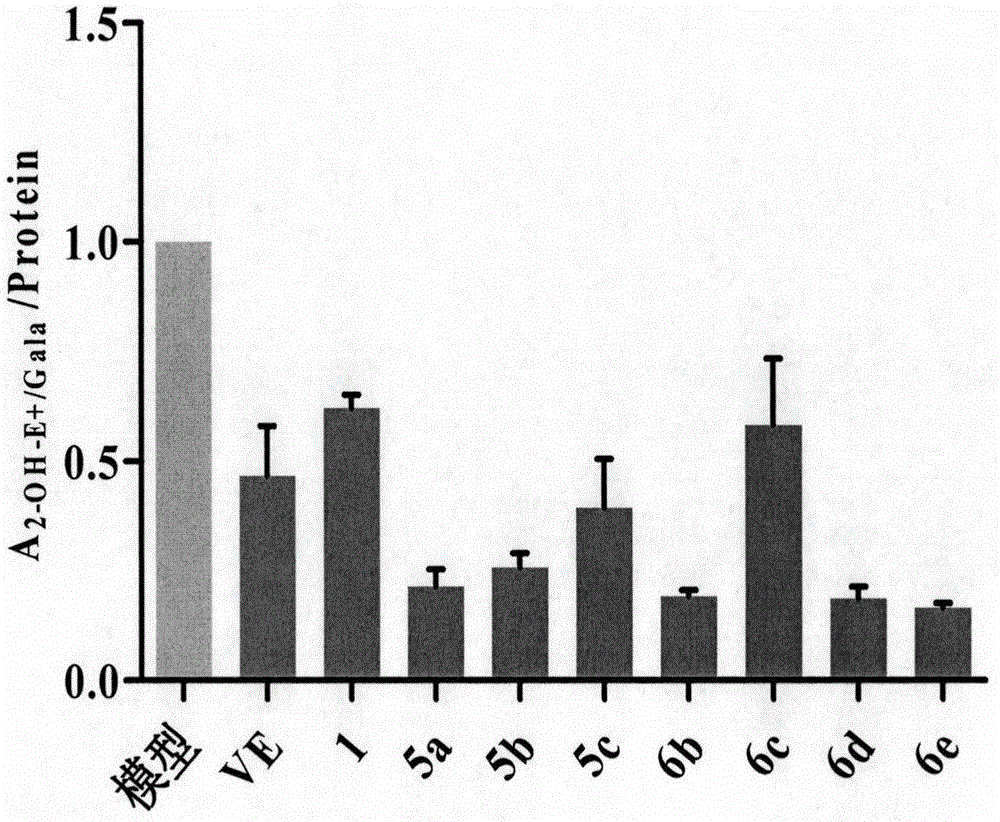
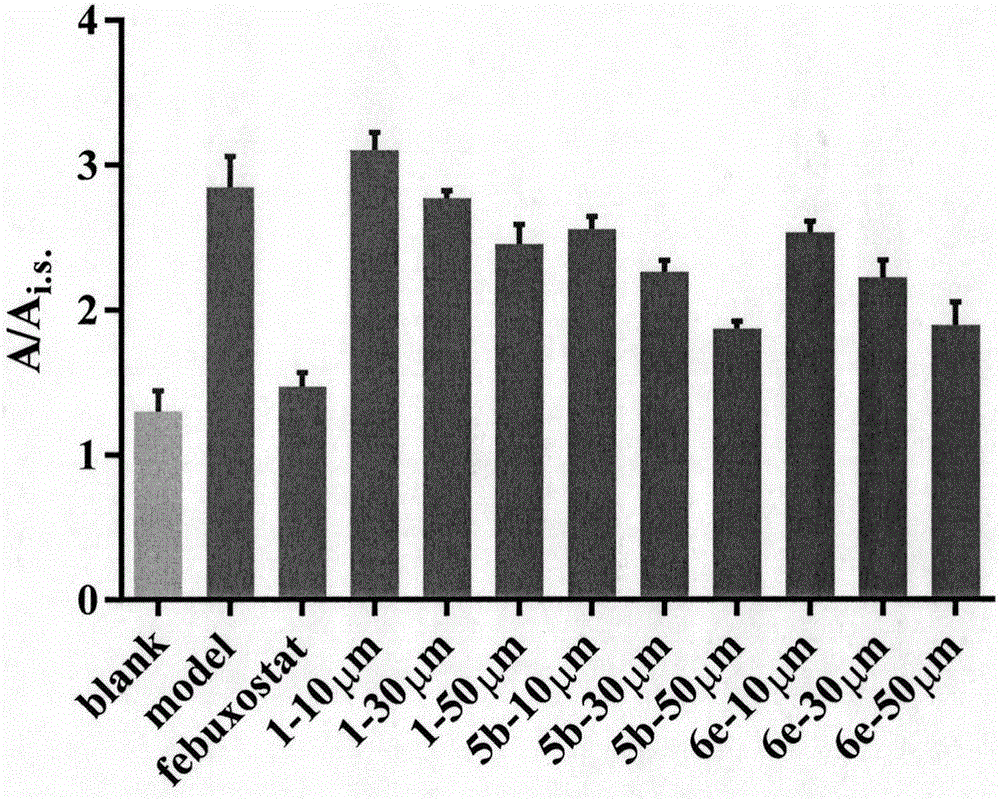
[0303] After mammalian macrophages phagocytize bacteria, aging degenerated cells, immune complexes or oxidized low-density lipoproteins, phagocytes enter a functionally activated state, the function of intracellular lysosomes is enhanced, the level of ROS is increased, and inflammatory cytokines are secreted and synthesized. Lipopolysaccharide (LPS) is the main component of the cell wall of Gram-negative bacteria and the main material basis for its pathogenicity. It can stimulate monocytes / macrophages to produce and release a large amount of reactive oxygen species (mainly including O 2 -· and H 2 o 2 ), nitric oxide (NO), IL-1β, and tumor necrosis factor-α (Tumor necrosis factor-α, TNF-α) and other inflammatory factors participate in the acute phase response of the body, causing inflammatory damage to the body. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com