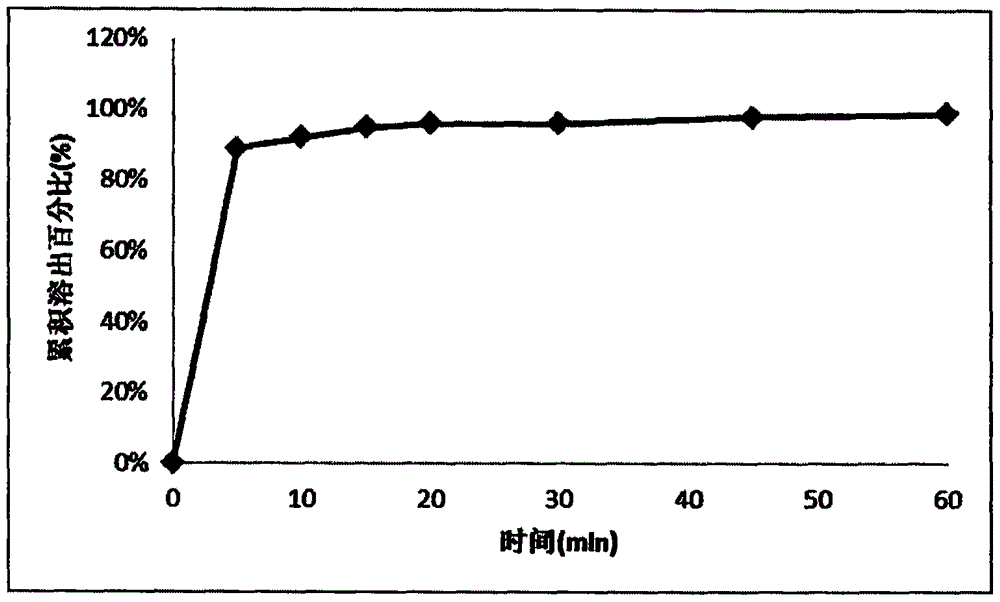
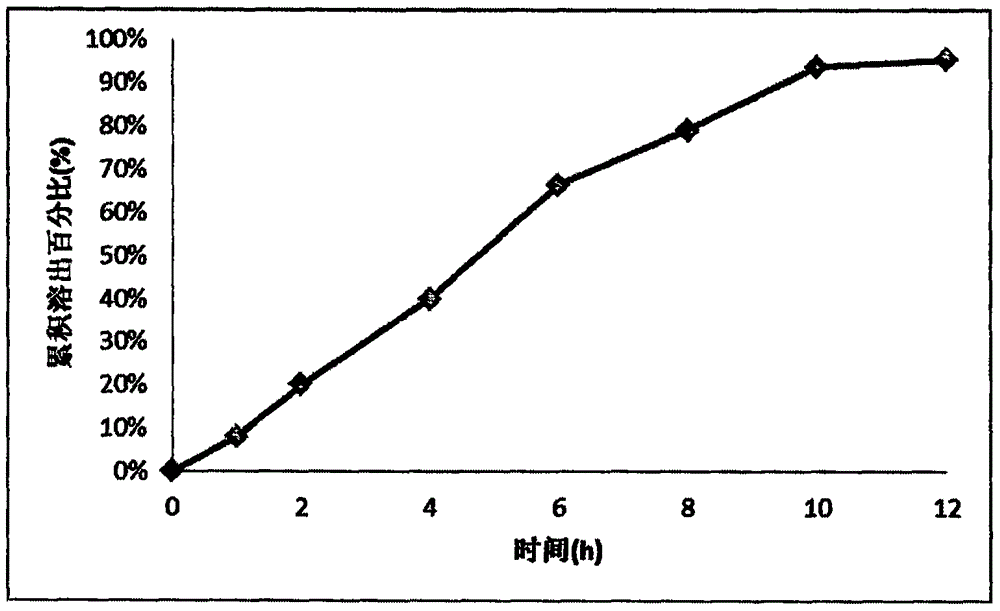
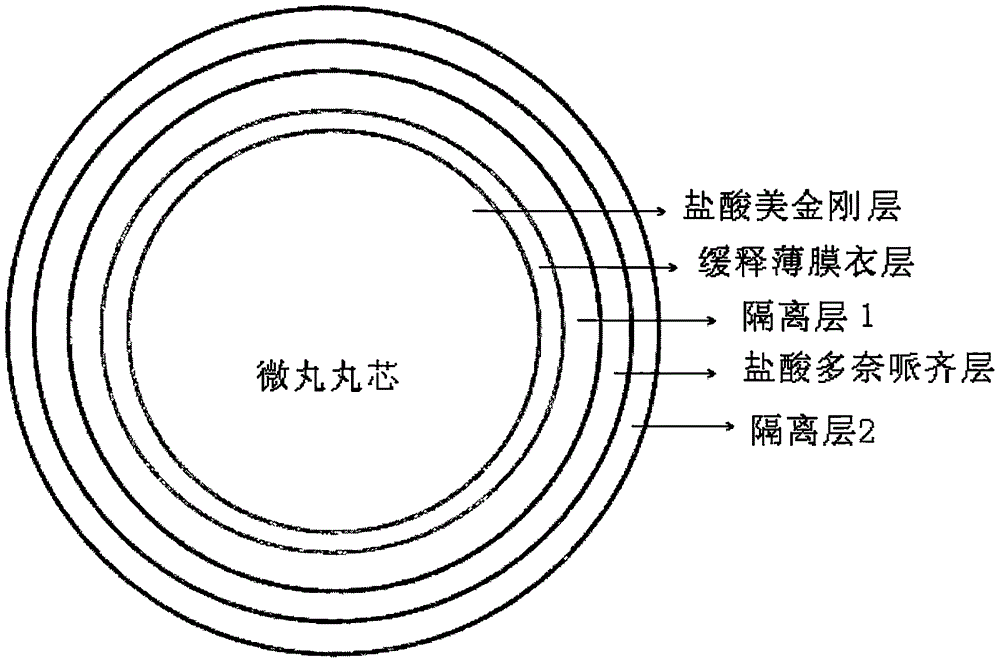
Memantine hydrochloride and donepezil hydrochloride composite preparation
A technology of memantine hydrochloride and donepezil, which is applied in the fields of pharmaceutical formulations, neurological diseases, capsule delivery, etc., can solve the problems of industrial scale-up difficulties, production workshops that cannot meet the requirements, and high requirements for capsule filling equipment, so as to avoid uneven filling , to avoid the effect of special requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A compound preparation of memantine hydrochloride donepezil, prepared from the following components,
[0049] Memantine Hydrochloride 14.0g
[0050] Donepezil Hydrochloride 10.0g
[0051] Microcrystalline Cellulose Pellet Core 70.0g
[0052] Ethyl cellulose aqueous dispersion 24.0g
[0053] Povidone 6.0g
[0054] Opadry 3.3g
[0055] Its preparation method comprises the following steps:
[0056] 1) memantine hydrochloride and povidone (3.0g) are dissolved in 50% ethanol solvent, wrapped on the surface of the microcrystalline cellulose pellet core,
[0057] 2) adopt ethylcellulose aqueous dispersion to carry out the coating of slow-release layer,
[0058] 3) Opadry (1.3g) was added with water to configure an 8% aqueous solution, and wrapped on the surface of the pellets as the isolation layer 1,
[0059] 4) donepezil hydrochloride and povidone (3.0g) are dissolved in a solvent of 50% ethanol, wrapped on the next layer of microcrystalline cellulose pellet core, as ...
Embodiment 2
[0069] A compound preparation of memantine hydrochloride donepezil, prepared from the following components,
[0070] Memantine Hydrochloride 14.0g
[0071] Donepezil Hydrochloride 10.0g
[0072] Microcrystalline cellulose pellet core 62.0g
[0073] Ethylcellulose aqueous dispersion 32.0g
[0074] Povidone 6.0g
[0075] Opadry 3.3g
[0076] Its preparation method comprises the following steps:
[0077] 1) memantine hydrochloride and povidone (3.0g) are dissolved in 50% ethanol solvent, wrapped on the surface of the microcrystalline cellulose pellet core,
[0078] 2) adopt ethylcellulose aqueous dispersion to carry out the coating of sustained-release layer,
[0079] 3) Opadry (1.3g) was added with water to be configured into an 8% aqueous solution, and wrapped on the surface of the pellets as the isolation layer 1,
[0080] 4) donepezil hydrochloride and povidone (3.0g) are dissolved in a solvent of 50% ethanol, wrapped on the next layer of microcrystalline cellulose pel...
Embodiment 3
[0089] A compound preparation of memantine hydrochloride donepezil, prepared from the following components,
[0090] Memantine Hydrochloride 14.0g
[0091] Donepezil Hydrochloride 10.0g
[0092] Microcrystalline Cellulose Pellet Core 66.0g
[0093] Ethyl cellulose aqueous dispersion 28.0g
[0094] Povidone 6.0g
[0095] Opadry 3.3g
[0096] Its preparation method comprises the following steps:
[0097] 1) memantine hydrochloride and povidone (3.0g) are dissolved in 50% ethanol solvent, wrapped on the surface of the microcrystalline cellulose pellet core,
[0098] 2) adopt ethylcellulose aqueous dispersion to carry out the coating of sustained-release layer,
[0099] 3) Opadry (1.3g) was added with water to be configured into an 8% aqueous solution, and wrapped on the surface of the pellets as the isolation layer 1,
[0100] 4) donepezil hydrochloride and povidone (3.0g) are dissolved in a solvent of 50% ethanol, wrapped on the next layer of microcrystalline cellulose pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com