Method for efficiently producing hexagonal boron nitride and co-producing sodium metaborate from borax
A technology of hexagonal boron nitride and sodium metaborate, applied in the field of functional material preparation, can solve the problems of low boron conversion rate, increase time and cost, waste of resources, etc., and achieve the effects of high purity, reduced time and cost, and easy handling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Mix 4.0g of anhydrous borax and 4.8g of urea by ball milling, pass the mixture through a 200-mesh sieve, move the material under the sieve into a crucible, put it in a roasting furnace, pass in nitrogen protective gas, and heat up to 800°C at a rate of 3°C / min , kept at room temperature for 4 hours, and naturally cooled to room temperature to obtain 3.4 g of a roasted sample; ground, added 50 ml of deionized water, heated to 75 ° C in a water bath, kept stirring for 1.5 h, and filtered; the filter residue was dried at 80 ° C, moved into a crucible, and placed in a roasting furnace. Introduce nitrogen protective gas, heat up to 1200°C, keep warm for 2 hours, and cool naturally to room temperature to obtain hexagonal boron nitride with a yield of 84%; the filtrate is spray-dried and roasted at 500°C to obtain anhydrous sodium metaborate, Its yield was 86%.
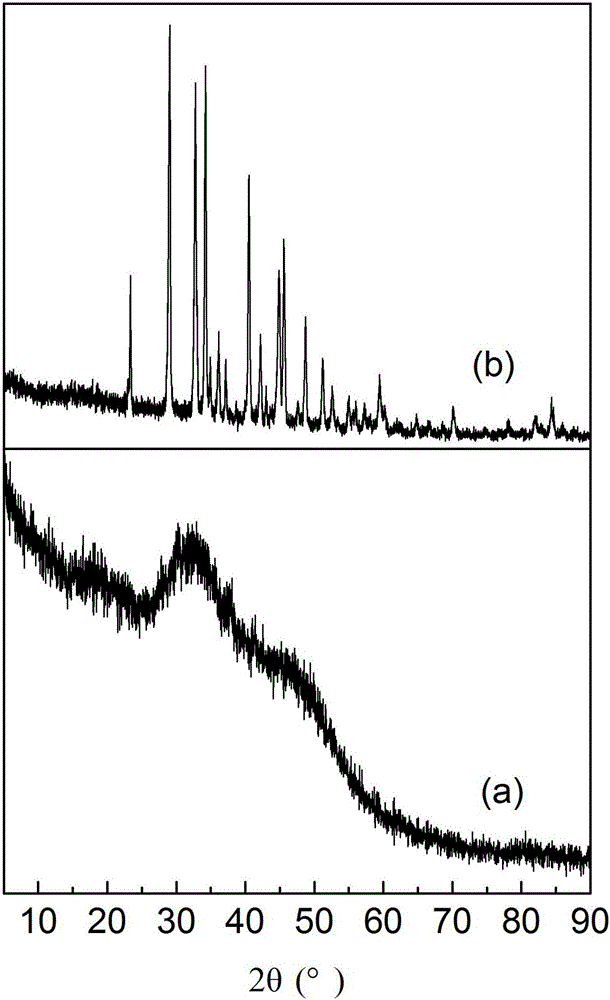
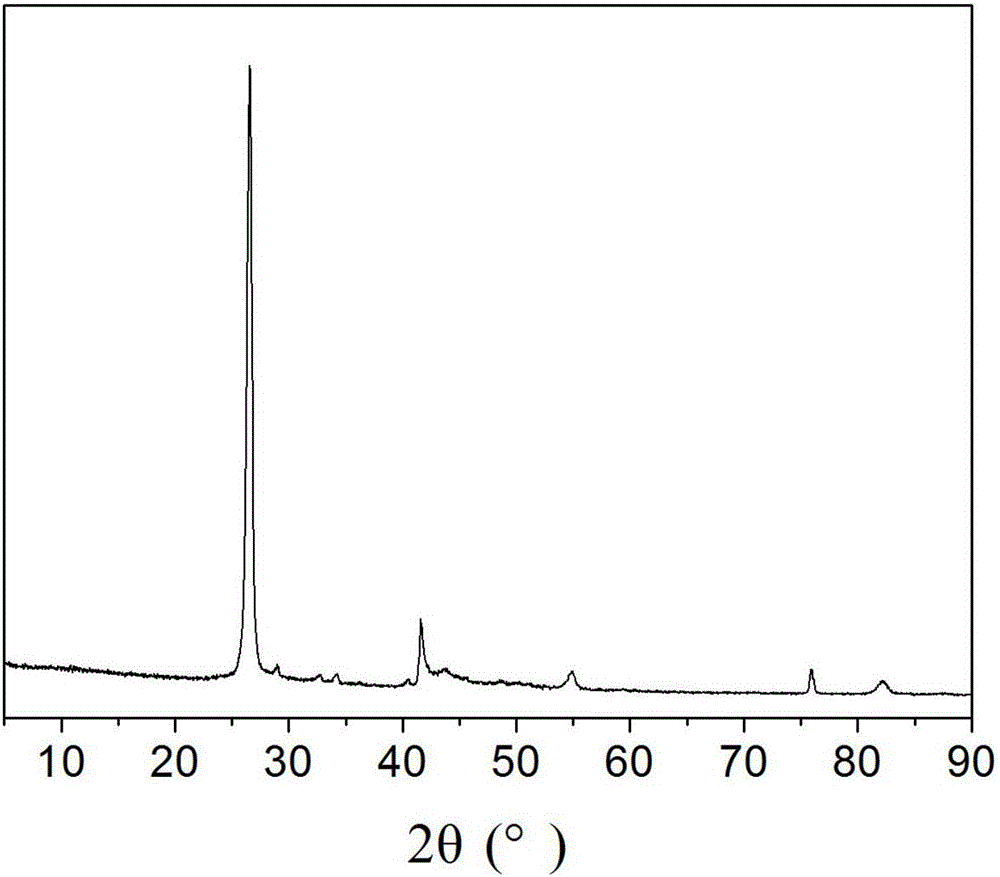
[0031] attached figure 1 And attached figure 2 It is the sample XRD spectrogram in this embodiment. figure 1 a ...
Embodiment 2
[0034] Mix 4.0g of anhydrous borax and 7.2g of urea by ball milling, pass the mixture through a 250-mesh sieve, move the material under the sieve into a crucible, put it in a roasting furnace, pass in nitrogen protective gas, and raise the temperature to 900°C at a rate of 4°C / min , kept at room temperature for 4 hours, and naturally cooled to room temperature to obtain 4.47 g of roasted samples; ground, added 112 ml of deionized water, heated to 80 ° C in a water bath, kept stirring for 1 h, and filtered; the filter residue was dried at 80 ° C and moved into a crucible, placed in a roasting furnace, and passed Enter nitrogen protective gas, heat up to 1300°C, keep warm for 4h, and naturally cool to room temperature to obtain hexagonal boron nitride with a yield of 85%; the filtrate is spray-dried and roasted at 600°C to obtain anhydrous sodium metaborate, Its yield was 86%.
[0035] In this embodiment, the comprehensive utilization rate of boron is 85%.
Embodiment 3
[0037] Mix 4.0g of anhydrous borax and 5.3g of ammonium chloride by ball milling, pass the mixture through a 300-mesh sieve, move the material under the sieve into a crucible, place it in a roasting furnace, feed nitrogen protective gas, and heat up to 900°C, keep warm for 3h, cool down to room temperature naturally, and get 3.5g roasted sample; Grind, add 70ml deionized water, heat in water bath to 70°C, keep stirring for 1h, filter; filter residue is dried at 80°C, moved into crucible, and placed in roasting furnace , into a nitrogen protective gas, heated to 1250°C, kept warm for 3 hours, and naturally cooled to room temperature to obtain hexagonal boron nitride with a yield of 80%; the filtrate was spray-dried and roasted at 650°C to obtain anhydrous metaboric acid Sodium, the yield was 82%.
[0038] In this embodiment, the comprehensive utilization rate of boron is 81%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com