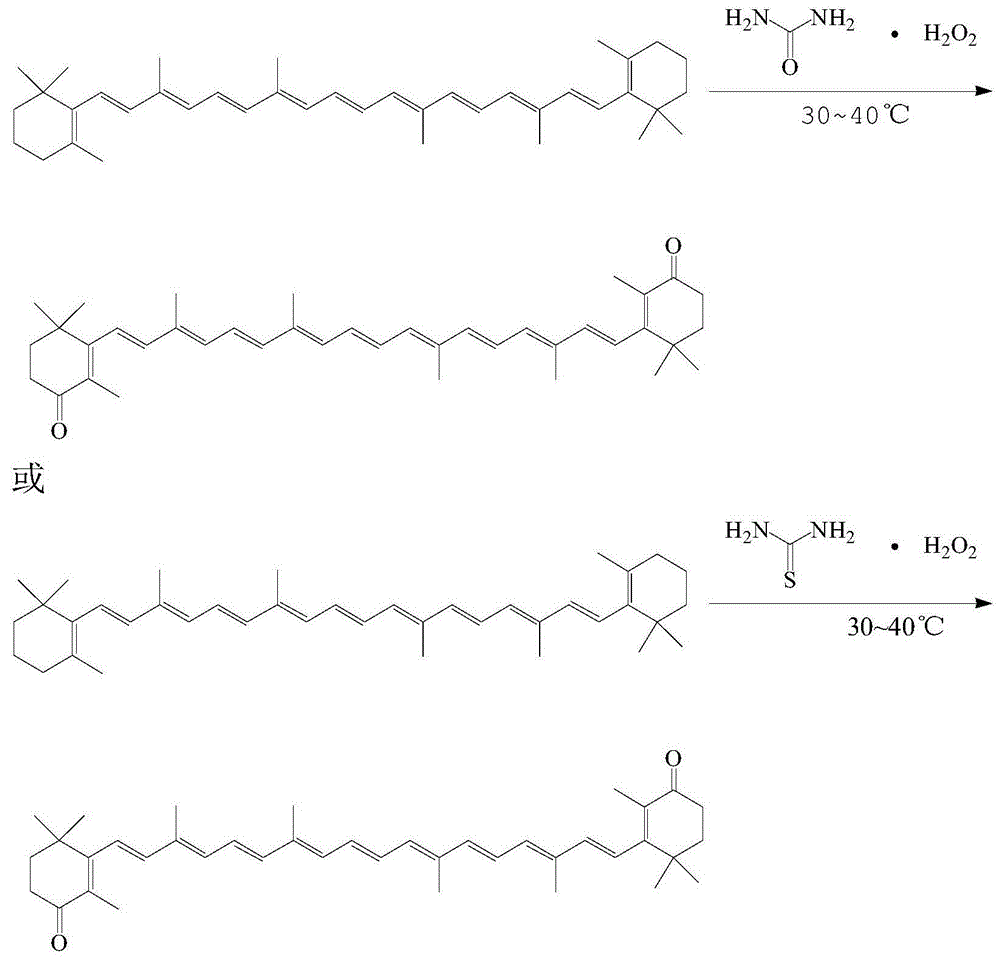
Method for preparing canthaxanthin through oxidation
A technology of canthaxanthin and oxidation method, applied in the direction of organic chemistry, etc., can solve problems such as difficulty in causing oxidation, unstable process, etc., and achieve the effects of good stability, stable reaction, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take 26.8g (0.05mol) of β-carotene and put it into a 1000ml four-necked flask, add 600ml of dichloromethane and stir to dissolve, then put the four-necked flask into a water bath to heat; add 21.6g (0.2mol) of thiourea peroxide Put it into a 500ml beaker, add 200ml of water, dissolve at room temperature, and transfer it to a 250ml constant pressure dropping funnel after the dissolution is complete. When the temperature in the four-neck flask rises to 30°C, start to add the thiourea peroxide solution dropwise, and the dropping time is controlled at about 1 hour. During the dropping process, the temperature must not exceed 40°C. After the dropping is completed, keep the reaction at 30°C for 8 hours. The organic layer was separated, washed and extracted three times with water, and the organic layer was separated after washing. The organic layer was rotary evaporated, and the solvent was completely removed to obtain 25 g of crude oil. Under HPLC conditions, with AE.LICHROM...
Embodiment 2
[0029] Take 26.8g (0.05mol) of β-carotene and put it into a 1000ml four-necked flask, add 600ml of dichloromethane and stir to dissolve, then put the four-necked flask into a water bath to heat; add 21.6g (0.2mol) of thiourea peroxide Put it into a 500ml beaker, add 200ml of water, dissolve at room temperature, and transfer it to a 250ml constant pressure dropping funnel after the dissolution is complete. When the temperature in the four-neck flask rises to 35°C, start to add thiourea peroxide solution dropwise, and the dropping time is controlled at about 1 hour. During the dropping process, the temperature must not exceed 40°C. After dropping, keep the reaction at 38°C for 16 hours. The organic layer was separated, washed and extracted three times with water, and the organic layer was separated after washing. The organic layer was rotary evaporated, and the solvent was completely removed to obtain 27.3 g of crude oil. Dissolve the crude oil in 300ml of ethanol, heat up to f...
Embodiment 3
[0031] Take 26.8g (0.05mol) of β-carotene and put it into a 1000ml four-necked flask, add 600ml of dichloromethane and stir to dissolve, then put the four-necked flask into a water bath to heat; add 21.6g (0.2mol) of thiourea peroxide Put it into a 500ml beaker, add 200ml of water, dissolve at room temperature, and transfer it to a 250ml constant pressure dropping funnel after the dissolution is complete. When the temperature in the four-neck flask rises to 35°C, start to add thiourea peroxide solution dropwise, and the dropping time is controlled at about 1 hour. During the dropping process, the temperature must not exceed 40°C. After dropping, keep the reaction at 38°C for 16 hours. The organic layer was separated, washed and extracted three times with water, and the organic layer was separated after washing. The organic layer was rotary evaporated, and the solvent was completely removed to obtain 27.1 g of crude oil. Dissolve the crude oil in 300ml of methanol, heat up to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com