Preparation method of double terminated glycol ether
A technology of glycol ether and ethylene glycol monoether, applied in the field of preparation of double-terminated glycol ether, can solve the problems of low catalyst yield, selectivity and service life, difficult regeneration of resin catalyst, etc. Large and small scale, selective improvement, high economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
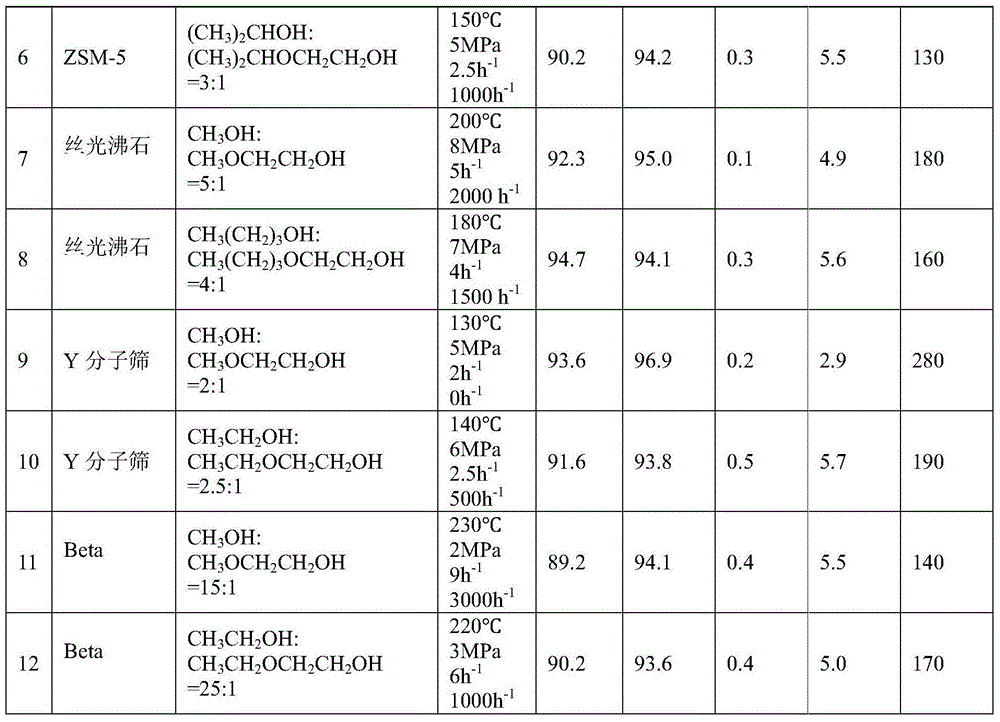
[0049] 50g of hydrogen-type MCM-22 molecular sieve catalyst with a silicon-aluminum ratio (Si:Al)=45:1 was calcined at 550°C for 5 hours in the air atmosphere of a muffle furnace, and a part of the powder sample was taken, pressed into tablets, and crushed into 20-40 mesh , for activity testing. Weigh 10 g of the hydrogen-type MCM-22 molecular sieve catalyst sample, put it into a stainless steel reaction tube with an inner diameter of 8.5 mm, activate it with nitrogen at normal pressure and 550 ° C for 4 hours, and then drop it to the reaction temperature (abbreviated as T) = 50 ° C , the molar composition of the feed material is CH 3 OH:CH 3 OCH 2 CH 2 OH=1:1, reaction pressure (abbreviated as P)=0.1MPa, mass space velocity of ethylene glycol monoether in the raw material (abbreviated as WHSV)=0.01h -1 , without carrier gas, the product was analyzed by gas chromatography. After the reaction was stable, the conversion rate of ethylene glycol monoether and the selectivity o...
Embodiment 2
[0051] The reaction conditions in Example 1 are changed to T=90°C, P=0.9MPa, and the molar composition of the feed material is CH 3 CH 2 OH:CH 3 CH 2 OCH 2 CH 2 OH=2:1, WHSV=0.5h -1 , Carrier gas nitrogen volume space velocity (abbreviated as GHSV) = 100h -1 , all the other experimental procedures are consistent with Example 1, and the reaction conditions and results are shown in Table 1.
Embodiment 3
[0053] The catalyst in Example 1 is replaced by a hydrogen-type ferrierite molecular sieve, Si:Al=15:1, T=300°C, P=15MPa, and the molar composition of the feed material is CH 3 OH:CH 3 OCH 2 CH 2 OH=100:1, WHSV=15h -1 , the carrier gas is nitrogen, GHSV=10000h -1 , all the other experimental procedures are consistent with Example 1, and the reaction conditions and results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com