Cefaclor preparation and preparation method thereof
A technology for cefaclor and preparations, applied in the field of medicine, can solve the problems of complicated purification and crystallization treatment, complicated reaction conditions, poor product quality and the like, and achieves the effects of fast reaction rate, high reaction yield and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
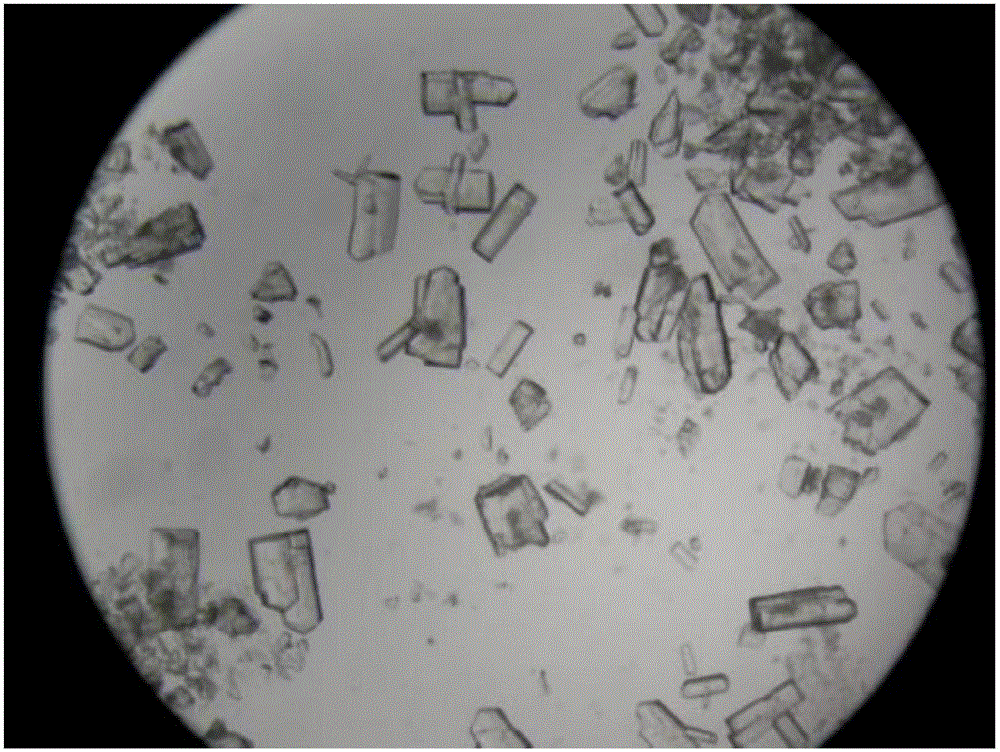
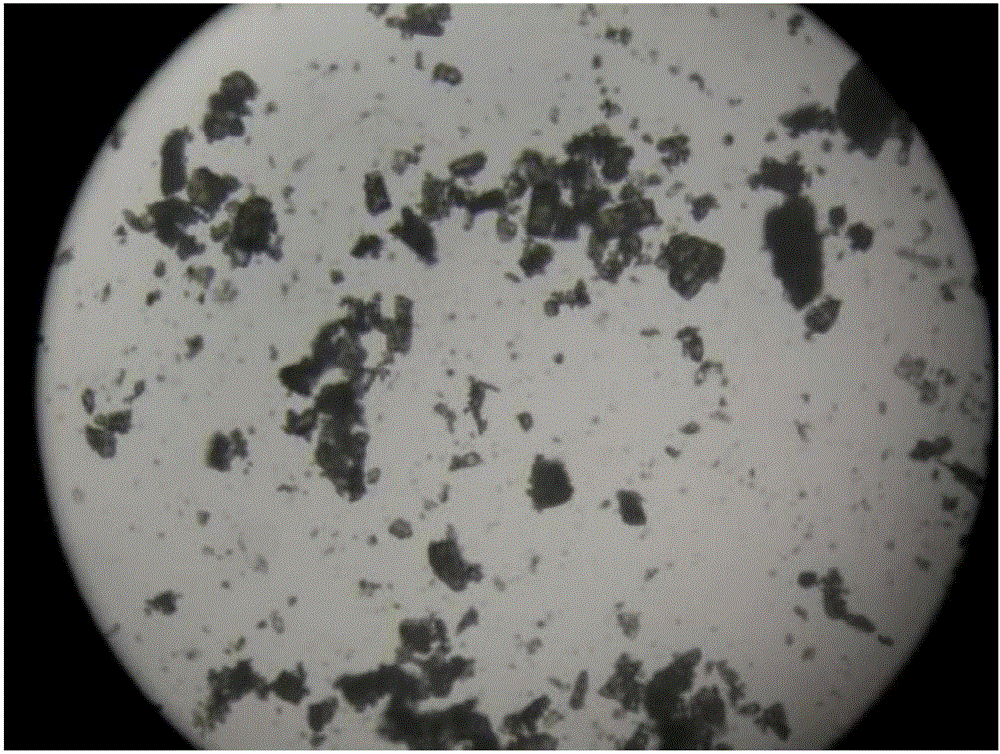
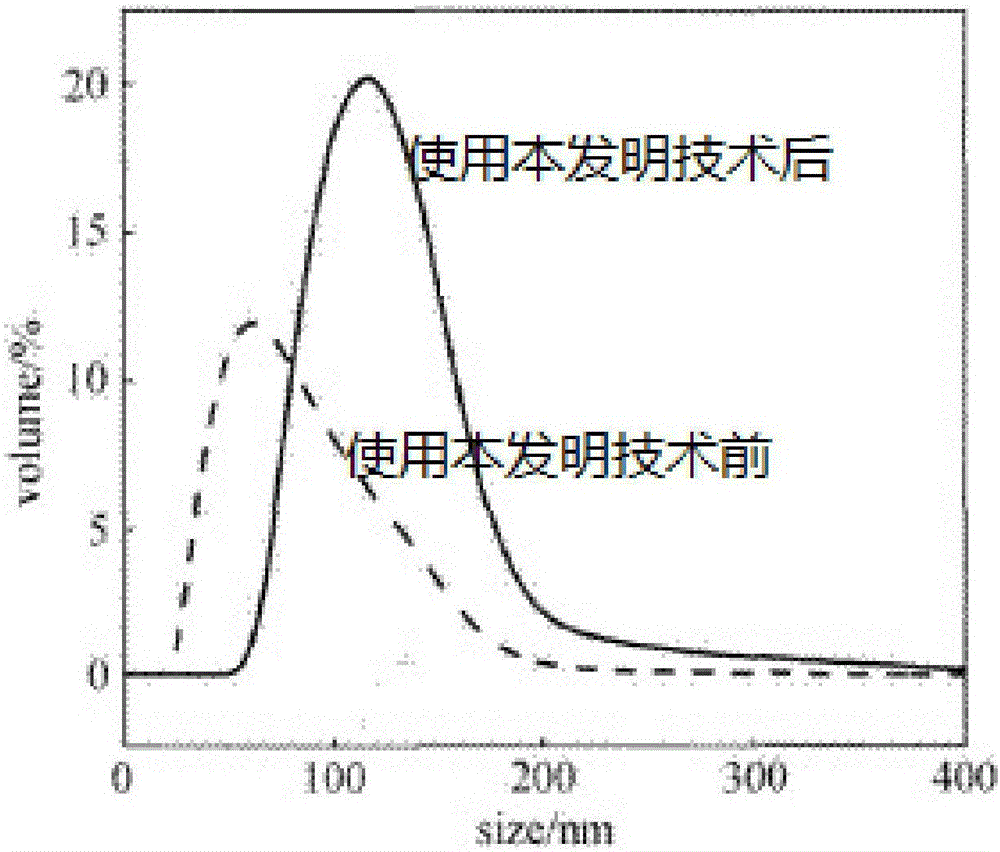
[0046] The invention discloses a preparation method of cefaclor preparation, comprising the preparation of cefaclor crystals, pretreatment, weighing, mixing, molding and packaging of main materials and auxiliary materials, and the preparation of cefaclor crystals is based on 7-ACCA, Deng potassium Salt is used as a raw material, and cefaclor crystals are prepared through silanization reaction, acylation reaction, condensation reaction, acidolysis reaction, extraction and impurity removal, decolorization and crystallization steps.
[0047] The preparation method of cefaclor crystals in the present invention specifically comprises the following processing steps,
[0048] (1) Silanization reaction: Add 7-ACCA to dichloromethane, dissolve at a temperature of 25-30°C to obtain a 7-ACCA solution, add a silylating reagent to the 7-ACCA solution, and react at a temperature of 40-46 The silanization reaction is carried out at ℃, and the temperature is lowered to -15℃ after the reaction...
Embodiment 1
[0063] This embodiment is a preparation example of cefaclor crystals, using 7-ACCA and Deng potassium salt as raw materials, wherein: the content of 7-ACCA (HPLC) ≥ 98.0%, moisture ≤ 1.0%, and the content of Deng potassium salt ≥ 99%, moisture ≤0.5%.
[0064] The preparation of cefaclor crystals includes seven steps of silanization reaction, acylation reaction, condensation reaction, acidolysis reaction, extraction and removal of impurities, decolorization and crystallization.
[0065] 1.1 Silanization reaction
[0066] Add 40ml of dichloromethane solvent to the reactor, control the temperature at 25°C, accurately weigh 20.00g of 7-ACCA and add it into the reactor to obtain a 7-ACCA solution, control the temperature at 25°C, and add the complex silylating reagent , the composite silylating reagent includes 6.33g trimethylchlorosilane, 3.60g hexamethyldisilazane, 0.083g N, O-bistrimethylsilylacetamide, control the reaction temperature at 40°C, start the heating and reflux reac...
Embodiment 2
[0080] This example is a preparation example of cefaclor crystals, using 7-ACCA and Deng potassium salt as raw materials, wherein: adopting 7-ACCA and Deng potassium salt as raw materials, wherein: the content of 7-ACCA (HPLC)≥98.0% , Moisture ≤ 1.0%, Deng potassium salt content ≥ 99%, moisture ≤ 0.5%.
[0081] The preparation of cefaclor crystals includes seven steps of silanization reaction, acylation reaction, condensation reaction, acidolysis reaction, extraction and removal of impurities, decolorization and crystallization.
[0082] 1.1 Silanization reaction
[0083] Add 100ml of dichloromethane solvent to the reactor, control the temperature at 25°C, accurately weigh 20.00g of 7-ACCA and add it to the reactor to obtain a 7-ACCA solution, control the temperature at 30°C, and add the complex silylating reagent , the composite silylating reagent includes 10.00g trimethylchlorosilane, 9.40g hexamethyldisilazane, 0.200g N, O-bistrimethylsilylacetamide, control the reaction t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com