Sarpogrelate hydrochloride tablet and preparation method thereof
A technology of sarcogrelate hydrochloride tablets and ester tablets, which is applied in the field of medicine, can solve the problems of large content of sarcogrelate hydrochloride, difficulty in ensuring the dissolution rate of preparations, and poor particle fluidity, so as to achieve low preparation cost and solve the problems of excipients and particle fluidity Contradictions, the effect of high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0029] Experimental example 1. Study on particle size control of raw and auxiliary materials of sarcogrelate hydrochloride tablets
[0030] 1. Study on the type of excipients
[0031] From the results of the compatibility test of raw materials and excipients, it is known that microcrystalline cellulose is most beneficial to the stability of sarcogrelate hydrochloride, so microcrystalline cellulose is selected as the excipient of the preparation, and the model is screened.
[0032] 1. Materials: sargrel hydrochloride (Hubei Jusheng Technology Co., Ltd., 20121101); microcrystalline cellulose PH101 (batch number: 1167), microcrystalline cellulose PH102 (batch number: 2193), microcrystalline cellulose PH301 (batch number: 3194 ), microcrystalline cellulose PH302 (batch number: 6114) were purchased from Asahi Kasei (China) Investment Co., Ltd. Beijing Branch.
[0033] 2. Method: Mix the above four different types of microcrystalline cellulose and sarcogrelate hydrochloride (crushe...
experiment example 2
[0075] Experimental example 2, citric acid adding process research
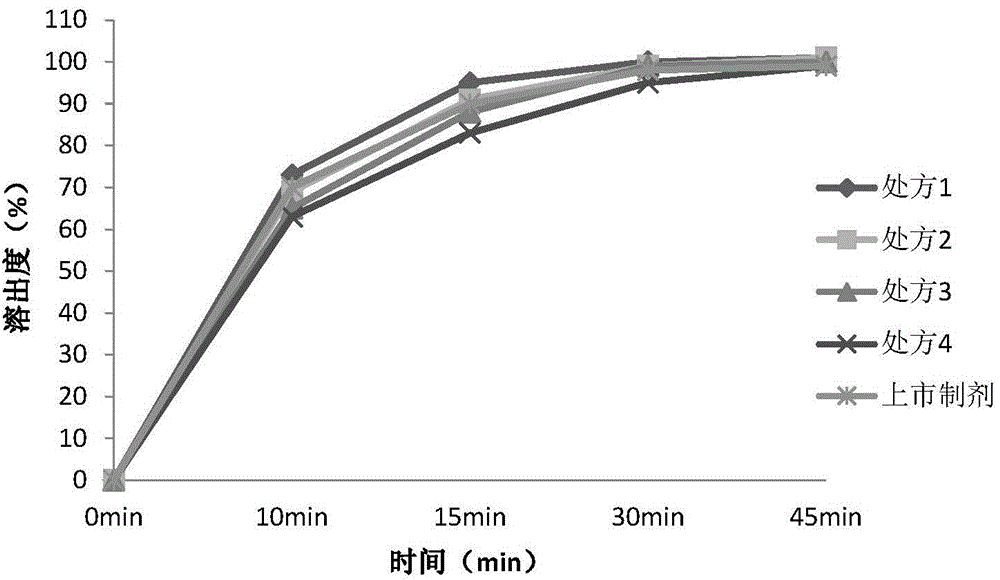
[0076] 1. Materials and instruments: sargrelate hydrochloride (Hubei Jusheng Technology Co., Ltd., batch number 20121101), microcrystalline cellulose PH302 (purchased from Asahi Kasei (China) Investment Co., Ltd. Beijing Branch, batch number: 6114), citric acid ( Company: Hunan Erkang Pharmaceutical Co., Ltd., batch number: 20120601), magnesium stearate (company: Shandong Liaocheng Ahua Pharmaceutical Co., Ltd., batch number: 20100214), micropowder silica gel (company: Shanghai Yunhong Chemical Pharmaceutical Co., Ltd., batch number: VA73913), 295F680010 (Company: Shanghai Kaleokang Coating Technology Co., Ltd., batch number: THL015076); pulverizer (model: F-30B, Jiangsu Baobao Group Co., Ltd.), tablet press (model: ZP-15, manufactured by Liaocheng Wanhe Industry Co., Ltd.), coating machine (model: BG10, Baoji Nuokai Technology Development Co., Ltd.). HPLC chromatograph (detector model: SPD-20A, pump model...
experiment example 3
[0100] Experimental Example 3, Stability Study of Sagrelate Hydrochloride Tablets
[0101] Carry out scale-up tests and stability studies in accordance with the determined prescription process, focusing on changes in substances related to accelerated tests of preparations.
[0102] 1. Materials and instruments: Anbu Leke (Company: Mitsubishi Tanabe Pharmaceutical Co., Ltd., Japan, batch number: U005), sargrelate hydrochloride (Hubei Jusheng Technology Co., Ltd., batch number 20121101), microcrystalline cellulose PH302 (purchased from Asahi Kasei (China) Investment Co., Ltd. Beijing Branch, batch number: 6114), citric acid (company: Hunan Erkang Pharmaceutical Co., Ltd., batch number: 20120601), magnesium stearate (company: Shandong Liaocheng Ahua Pharmaceutical Co., Ltd., Batch number: 20100214), micropowder silica gel (company: Shanghai Yunhong Chemical Pharmaceutical Co., Ltd., batch number: VA73913), 295F680010 (Company: Shanghai Kaleokang Coating Technology Co., Ltd., ba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com