Self-assembly antibacterial peptide
A self-assembly, antibacterial peptide technology, applied in the direction of antibacterial drugs, peptides, peptide/protein components, etc., can solve problems such as insufficient depth, and achieve the effects of increasing water solubility, good self-assembly ability, excellent antibacterial activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of self-assembling antimicrobial peptides:
[0028] 1. Materials
[0029] (1) Take by weighing MBHA resin (toluene hydrogenamine resin) 0.7861g;
[0030] (2) Prepare a DMF (dimethylformamide) solution of the following substances at a concentration of 0.2mol / L:
[0031] Fmoc-Phe-OH (N-fluorenylmethoxycarbonyl-phenylalanine): volume 21mL, mass 1.63g;
[0032] Fmoc-Lys(Boc)-OH(N-fluorenylmethoxycarbonyl-N'-tert-butoxycarbonyl-lysine): volume 21mL, mass 1.97g;
[0033] Fmoc-Pro-OH (N-fluorenylmethoxycarbonyl-proline): volume 11mL, mass 0.74g;
[0034] Fmoc-Leu-OH (N-fluorenylmethoxycarbonyl-leucine): volume 21mL, mass 1.48g;
[0035] Fmoc-Gly-OH (N-fluorenylmethoxycarbonyl-glycine): volume 11mL, mass 0.65g;
[0036] Fmoc-Ala-OH (N-fluorenylmethoxycarbonyl-alanine): volume 11mL, mass 0.68g;
[0037] Fmoc-Arg(Pbf)-OH(N-Fremoxycarbonyl-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-arginine): volume 11mL, mass 1.43g;
[0038] Nap-CH 2 COOH: volume 11mL, m...
Embodiment 2
[0048] Self-assembled morphology and secondary structure of self-assembled antimicrobial peptides
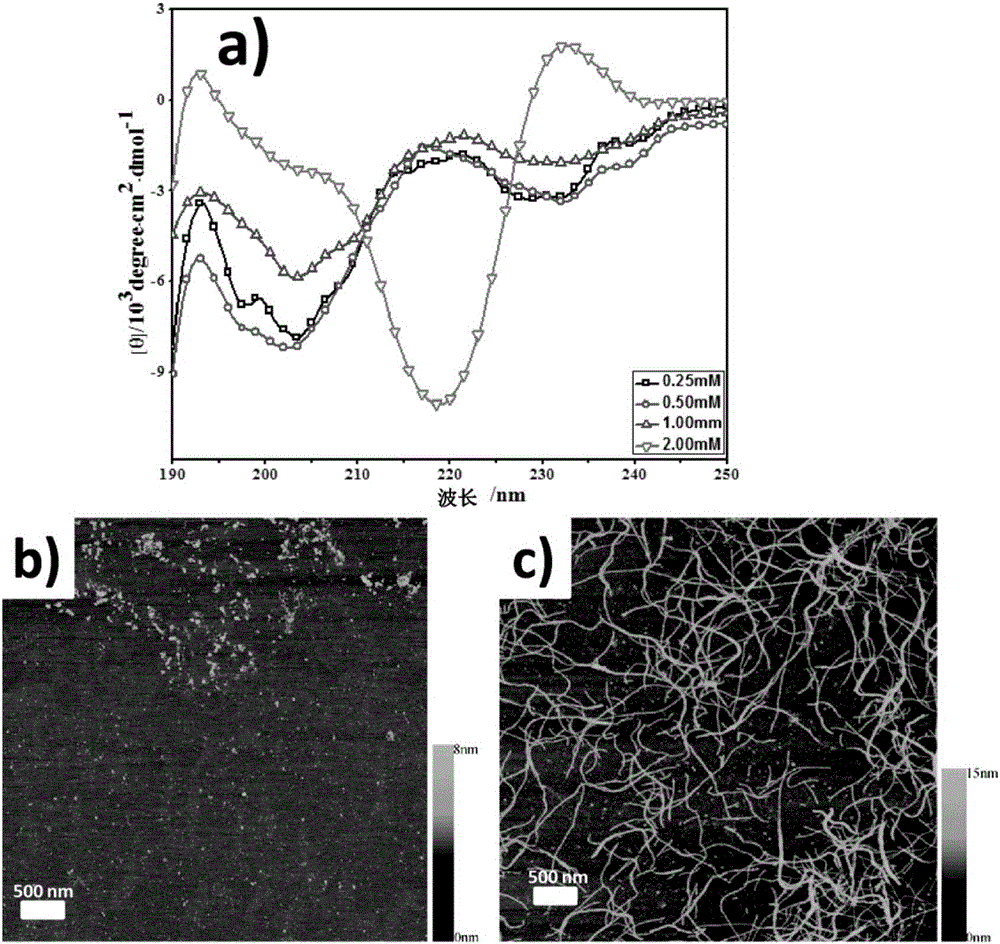
[0049] (1) Prepare the phosphate buffer solution (pH7.0, ionic strength 10mM) of the polypeptide molecule Nap-Phe-Phe-Lys-Pro-Leu-Gly-Leu-Ala-Arg-Lys, and its concentration is 0.25mM, 0.5mM respectively mM, 1.0mM and 2.0mM, placed at room temperature for 3 days, observed circular dichroism spectrum results and atomic force microscope results.
[0050] The results of the circular dichroism spectrum are as follows figure 1 As shown in a, it is found that the polypeptide molecule mainly presents a random coil secondary structure when the concentration is below 1.0 mM; at a concentration of 2.0 mM, it presents a β-sheet secondary structure.
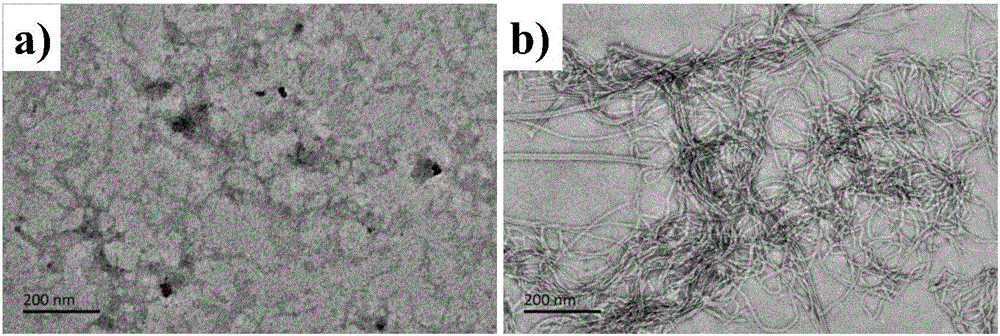
[0051] Atomic force microscopy results such as figure 1 b. figure 1 As shown in c, it was found that under the concentration of 1.0mM polypeptide molecules, there were many random aggregates in the solution, but no regular self-assembly stru...
Embodiment 3
[0056] Cell / bacterial culture test
[0057] (1) Cell experiment: first inoculate 100 μl in a sterile 96-well plate with a density of 1×10 5 Cells / mL HeLa / NIH3T3 cells were placed in a 37°C incubator for 24 hours. After they adhered to the wall, the culture solution in the well plate was sucked out, and 100 μl of fresh culture solution and 100 μl of filtered different concentrations of Polypeptide solution, its absorbance is Abs peptide , 4 parallels were set up for each concentration, and the wells that only added Tris (trishydroxymethylaminomethane) buffer and no peptide were used as a control group, and the absorbance was Abs Tris , and then put the well plate back in the incubator at 37°C for 6h. After the effect was completed, 20 μl of MTT (3-(4,5-dimethylthiazole-2)-2 at a concentration of 5 mg / mL was added to each well. , 5-diphenyltetrazolium bromide) solution, continue culturing in the incubator for 4 hours, then suck out the liquid in the well plate, add 150 μl of d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com