Peptoid inhibitor as well as preparation method and application thereof
A technology of peptide inhibitors and inhibitors, applied in the field of biomedicine, can solve the problems of complicated preparation steps and difficult industrial production, and achieve the effect of reducing toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0051] Preparation of Experimental Example 1 Peptide Inhibitor API1
[0052] The peptide inhibitor API1 of the present invention is synthesized by a solid-phase subunit synthesis method, the method comprising the following steps:
[0053] (1) Add 2M bromoacetic acid and 3.2M N,N'-diisopropylcarbodiimide (DIC) to Rink amide AM resin (substitution level 0.3mmol / g), react at 37°C for 30min, and the resin end Amino acylation;
[0054] (2) Add 2M primary amine and react at 37°C for 90 minutes, and replace the bromine atom by nucleophilic displacement reaction to complete the synthesis of a subunit;
[0055] (3) Steps (1) and (2) are repeated until the synthesis of the remaining subunits is completed;
[0056] (4) After the synthesis is completed, the side chain protecting group is removed, and API1 is cleaved from the resin with 95% trifluoroacetic acid, 2.5% ultrapure water, and 2.5% triisopropylsilane for future use.
[0057] The molecular formula of the prepared API1 is as fo...
experiment example 2
[0061] Experimental Example 2 Changes in the morphology of β-amyloid polypeptide Aβ1-42 aggregates by the peptide inhibitor API1
[0062] The β-amyloid polypeptide Aβ1-42 was dissolved in phosphate buffer and incubated with the peptoid inhibitor API1 for 48 hours at 37°C with constant temperature shaking, and the effect of API1 on the aggregation morphology of Aβ1-42 was observed by atomic force microscopy. The characterization of the influence of API1 on the aggregation morphology of Aβ1-42 comprises the following steps:
[0063] (1) Dissolving Aβ1-42 in phosphate buffer solution with pH 7.2 to make a 20 μM solution;
[0064] (2) API1 and Aβ1-42 were mixed equimolarly, dissolved in phosphate buffer solution of pH 7.2 to prepare a mixed buffer solution, the concentrations were respectively 200 μM and 20 μM, and co-incubated at 37° C. for 24 hours;
[0065] (3) Add the mixed solutions obtained in steps (1) and (2) dropwise onto flat substrates such as mica and silicon wafers, ...
experiment example 3
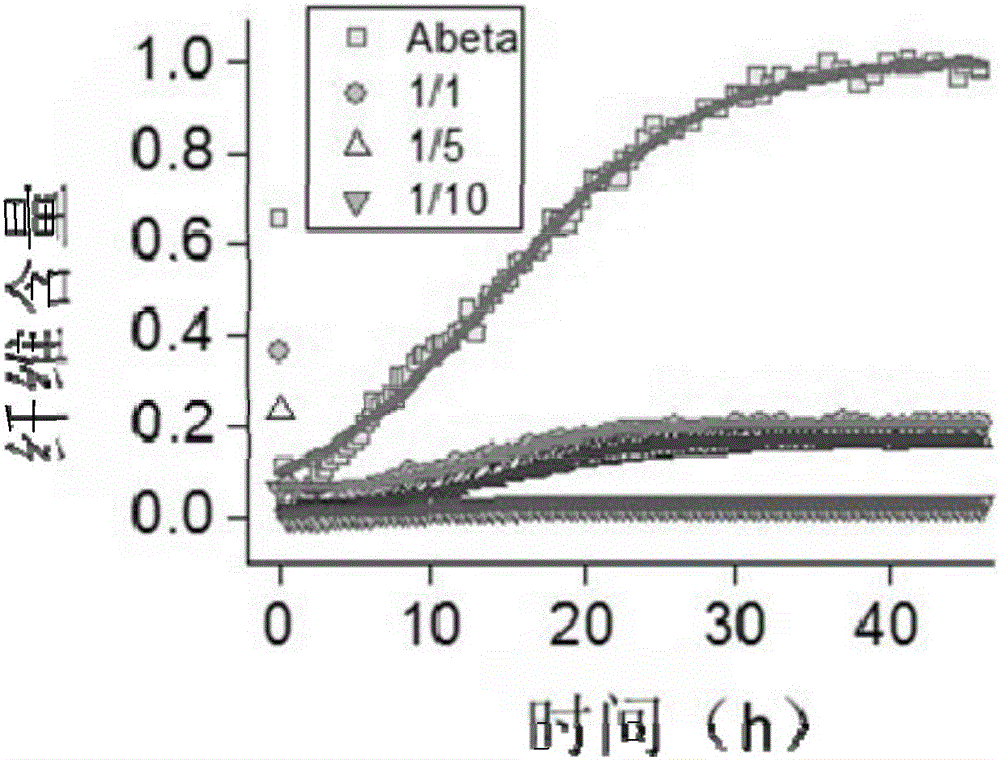
[0067] Experimental Example 3 Aggregation Inhibitory Effect of Peptide Inhibitor API1 on Aβ1-42 Polypeptide
[0068] The specific steps for detecting the aggregation inhibitory effect of the peptide inhibitor API1 of the present invention on the Aβ1-42 polypeptide using ThT fluorescence technology are as follows:
[0069] (1) Prepare 20 μM Aβ1-42 solution, Aβ1-42:API1=1:1, 1:5 and 1:10 mixed buffer;
[0070] (2) adding THT fluorescent dye to the solution configured in step (1);
[0071] (3) Test the mixed solutions obtained in step (2) respectively. Select a specific excitation wavelength and emission wavelength, and reflect the aggregation degree of Aβ1-42 in the solution by testing the fluorescence intensity at the emission wavelength.
[0072] The experimental condition is 37°C, and the test is performed every 15 minutes. The results are as follows: figure 2 As shown, API1:Aβ1-42=1:1, 1:5, and 1:10 respectively gradually strengthened the aggregation inhibitory effect on ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com