Method for separating and determining 1,2-propylene glycol enantiomers by using gas chromatography
A technology of gas chromatography and enantiomers, which is applied in the field of separation and determination of 1,2-propanediol enantiomers by pre-column derivatization gas chromatography, can solve the problems of inability to accurately quantify trace impurities and tailing, etc. Achieve the effects of improving tailing phenomenon, fast reaction speed and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Instruments and Conditions
[0035] Shimadzu gas chromatograph (GC-2010) and LabSolutions workstation; automatic sample injection; CD-Chirasil-DEXCB (0.25 μm, 25m×0.25 μm) as the analytical column; the detector is a hydrogen flame ion detector; the initial temperature is 60 ℃, maintained for 10 minutes, raised to 150 ℃ at a rate of 40 ℃ per minute, and maintained for 5 minutes; the temperature of the injection port was 250 ℃, and the split ratio was 50:1; the temperature of the detector was 250 ℃; the carrier gas was nitrogen, and the flow rate was It is 1.0ml per minute, and the injection volume is 1ml.
[0036] Experimental procedure
[0037] Precisely measure 5ml of acetone and put it into a microreactor, add 0.5g of water remover (4A molecular sieve) and 10mg of catalyst (Amberlyst-15), seal it, stir at room temperature for 1h, take the reaction solution and filter it with 0.45μm, and the filtrate is used as the test sample solution. Get need testing solution and...
Embodiment 2
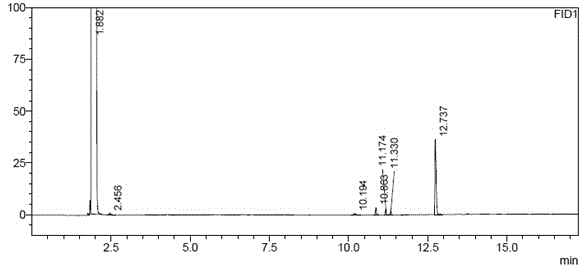
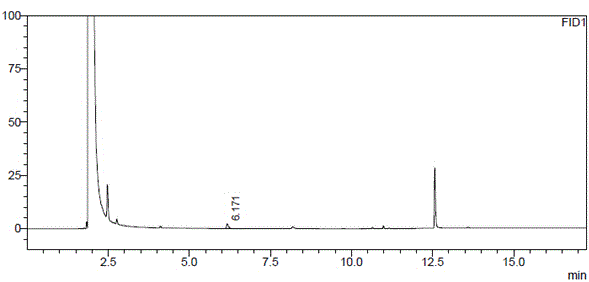
[0042] Take about 100mg of (R)-1,2-propanediol, accurately weigh it, put it in a 10ml measuring bottle, add acetone to dissolve and dilute to the mark, shake well, accurately measure 5ml and place it in a microreactor, add a water remover (4A Molecular sieve) 0.5g and catalyst (Amberlyst-15) 10mg, seal, stir at room temperature for 1h, take the reaction solution and filter it with 0.45μm, and the filtrate is used as the test solution. Get need testing solution and carry out gas chromatograph analysis under above-mentioned conditions, the results are shown in Figure 5 ; The results of the determination of (S)-1,2-propanediol by the same method are shown in Figure 6 .
[0043] Figure 5 The chromatographic peaks with retention times of 6.0 minutes and 7.0 minutes are (R)-1,2-propanediol derivatives and their enantiomer impurities (S)-1,2-propanediol derivatives (impurity content: 0.96%), respectively. Figure 6 The chromatographic peaks at 6.6 minutes and 6.4 minutes were (...
Embodiment 3
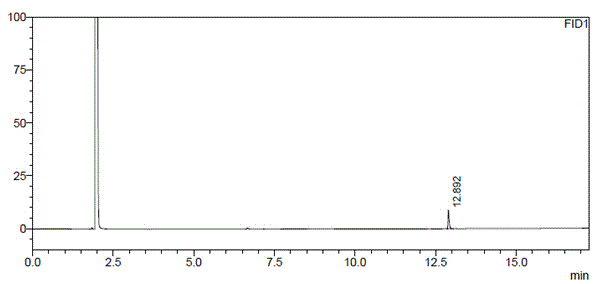
[0045] Take about 0.5g of dapagliflozin raw material (approximately equivalent to (S)-1,2-propanediol 90mg), weigh it accurately, put it in a 10ml measuring bottle, add acetone to dissolve and dilute to the mark, shake well, and measure it precisely Put 5ml into a microreactor, add 0.5g of water remover (4A molecular sieve) and 20mg of catalyst (Amberlyst-15), seal it, stir at room temperature for 1h, take the reaction solution and filter it with 0.45μm, and the filtrate is used as the test solution; (R)-1,2-propanediol reference substance is about 10mg, accurately weighed, put in a 10ml measuring bottle, add acetone to dissolve and dilute to the mark, shake well, accurately measure 5ml and put it in a micro reactor, add a water remover ( 4A molecular sieve) 0.5g and catalyst (Amberlyst-15) 20mg, seal, stir at room temperature for 1h, take the reaction solution and filter it with 0.45μm, accurately measure 1ml of the subsequent filtrate, put it in a 100ml measuring bottle, dilu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com