Method for determining content of clindamycin phosphate vaginal tablets
A clindamycin phosphate and assay method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of unclear separation of the main peak, large decrease in the content of the main peak, long retention time of the main peak, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The assay of embodiment 1 clindamycin phosphate vaginal tablet
[0034] HPLC detection conditions are as follows:
[0035] Stationary phase: octylsilane bonded silica gel;
[0036] Mobile phase A: Potassium dihydrogen phosphate-acetonitrile-water-heptafluorobutyric acid-ferric chloride, the volume ratio is 10:3:11:2:1;
[0037] Mobile phase B: a mass fraction of 0.1% aqueous formic acid;
[0038] Flow rate: 1.3ml / min;
[0039] Column temperature: 50°C;
[0040] Detection wavelength: 200nm;
[0041] The elution conditions are:
[0042] (1) From 0 to 5.0 minutes, the volume ratio of mobile phase A to mobile phase B is 40:60;
[0043] (2) From 5.01 to 9 minutes, the volume ratio of mobile phase A to mobile phase B is 55:45;
[0044] (3) From 9.01 to 25 minutes, the volume ratio of mobile phase A to mobile phase B is 75:25;
[0045] S1, get 8 clindamycin phosphate vaginal tablets (32mg), dissolve in ethanol after grinding, filter,
[0046] S2, measure the supernata...
Embodiment 2
[0048] The content determination of embodiment 2 clindamycin phosphate vaginal tablets
[0049] HPLC detection conditions are as follows:
[0050] Stationary phase: octylsilane bonded silica gel;
[0051] Mobile phase A: Potassium dihydrogen phosphate-acetonitrile-water-pentafluoropropionic acid-ferric chloride, the volume ratio is 15:8:15:4:3;
[0052] Mobile phase B: a mass fraction of 1.3% aqueous formic acid;
[0053] Flow rate: 1.5ml / min;
[0054] Column temperature: 70°C;
[0055] Detection wavelength: 400nm;
[0056] The elution conditions are:
[0057] (1) From 0 to 5.0 minutes, the volume ratio of mobile phase A to mobile phase B is 45:55;
[0058] (2) From 5.01 to 9 minutes, the volume ratio of mobile phase A to mobile phase B is 60:40;
[0059] (3) From 9.01 to 25 minutes, the volume ratio of mobile phase A to mobile phase B is 80:20;
[0060] S1, get 13 clindamycin phosphate vaginal tablets (52mg), dissolve in ethanol after grinding, shake well, filter,
...
Embodiment 3
[0063] The assay of embodiment 3 clindamycin phosphate vaginal tablets
[0064] HPLC detection conditions are as follows:
[0065] Stationary phase: Octylsilane bonded silica gel
[0066] Mobile phase A: Potassium dihydrogen phosphate-acetonitrile-water-trifluoroacetic acid-ferric chloride, the volume ratio is 13:5:12:3:2;
[0067] Mobile phase B: a mass fraction of 1.0% aqueous formic acid;
[0068] Flow rate: 1.4ml / min;
[0069] Column temperature: 60°C;
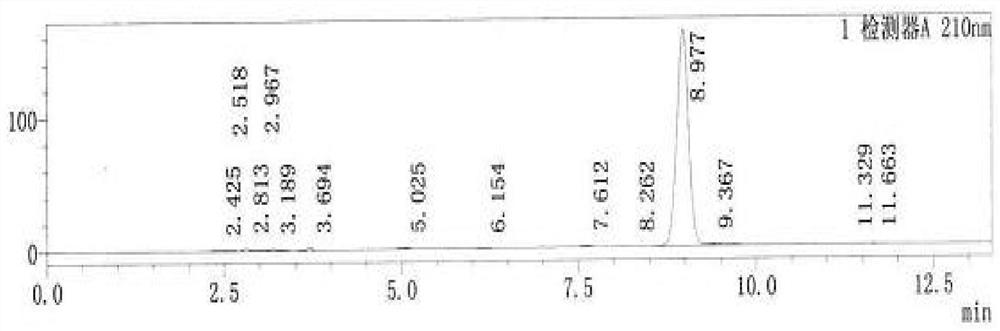
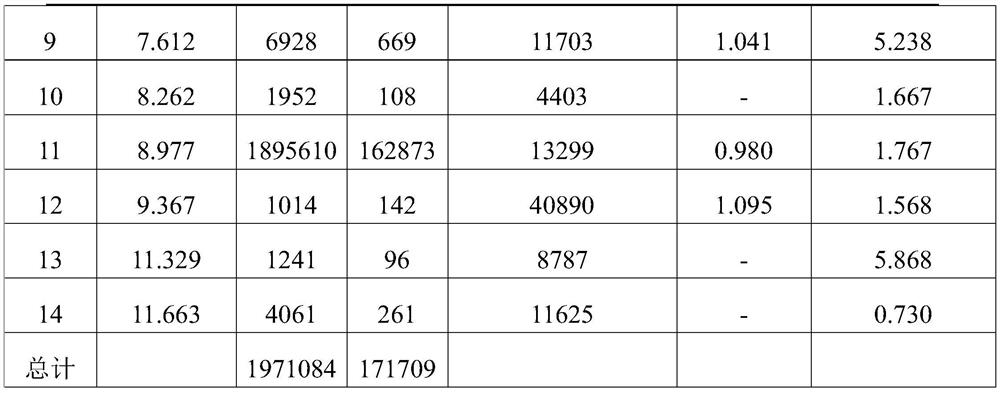
[0070] Detection wavelength: 210nm.
[0071] The elution conditions are:
[0072] (1) From 0 to 5.0 minutes, the volume ratio of mobile phase A to mobile phase B is 42:58;
[0073] (2) From 5.01 to 9 minutes, the volume ratio of mobile phase A to mobile phase B is 57:43;
[0074] (3) From 9.01 to 25 minutes, the volume ratio of mobile phase A to mobile phase B is 78:22;
[0075] S1, get 10 clindamycin phosphate vaginal tablets (40mg), dissolve in ethanol after grinding, filter,
[0076] S2, measure the supernatant 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com