Process for preparing unsaturated esters proceeding from aldehydes by direct oxidative esterification
A technology of oxidative esterification and methacrolein, applied in chemical instruments and methods, preparation of carboxylic acid esters, preparation of organic compounds, etc., can solve the problems of catalyst short life, achieve catalyst service life, improve yield, The effect of suppressing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
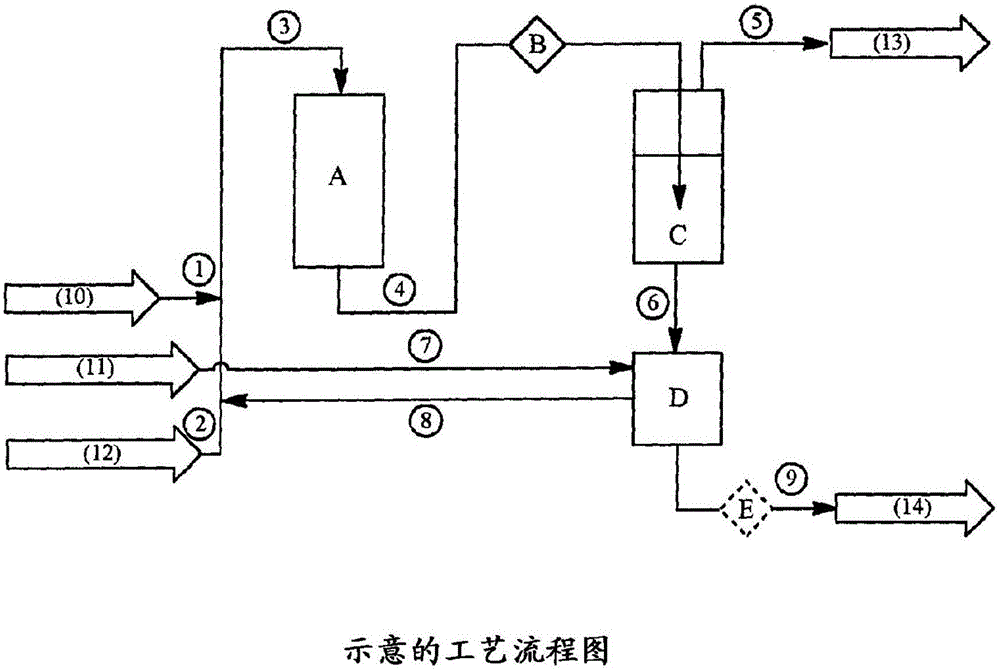
Image
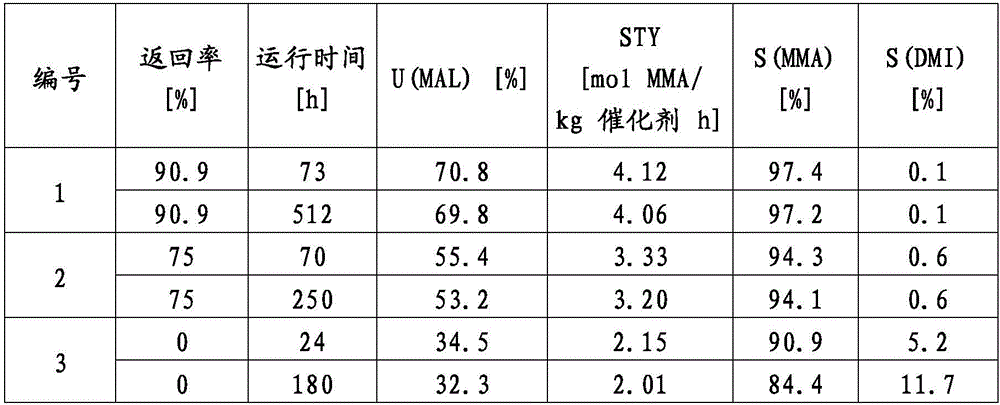
Examples
Embodiment
[0058] Catalyst preparation
[0059] Catalyst 1 (on SiO 2 -Al 2 o 3 -0.9%Au-1.1%NiO on MgO, 1.16-2.36mm balls)
[0060] A solution of 37.5g of aluminum nitrate nonahydrate, 25.6g of magnesium nitrate hexahydrate and 5.4g of 60% nitric acid in 100ml of water was mixed with 108g of SiO at room temperature. 2The carrier (FujiSilicia, CariactQ-10, 1.16-2.36 mm balls) was mixed. The mixture was stirred at 50°C for 24 hours, then cooled to room temperature, dried at 130°C and calcined at 300 to 600°C for a total of 10 hours. 30g of this SiO 2 -Al 2 o 3 - The MgO carrier is mixed with 100ml of water and heated to 90°C. After 15 minutes, 1.64 g of nickel nitrate hexahydrate and 530 mg of gold acid (HAuCl 4 ) in 100 ml of water was added to the mixture at 90° C. within 30 minutes. After stirring for a further 30 minutes at 90° C., the mixture was cooled and the solid separated, then stirred three times at 20° C. with 100 ml of fresh water for 5 minutes each and filtered. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com