n-5,6,7-hydroquinoline-8-arylimine nickel complex catalyst and its preparation method and application
A technology of catalysts and complexes, applied in nickel organic compounds, organic chemistry, etc., to achieve the effect of large industrial application potential and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
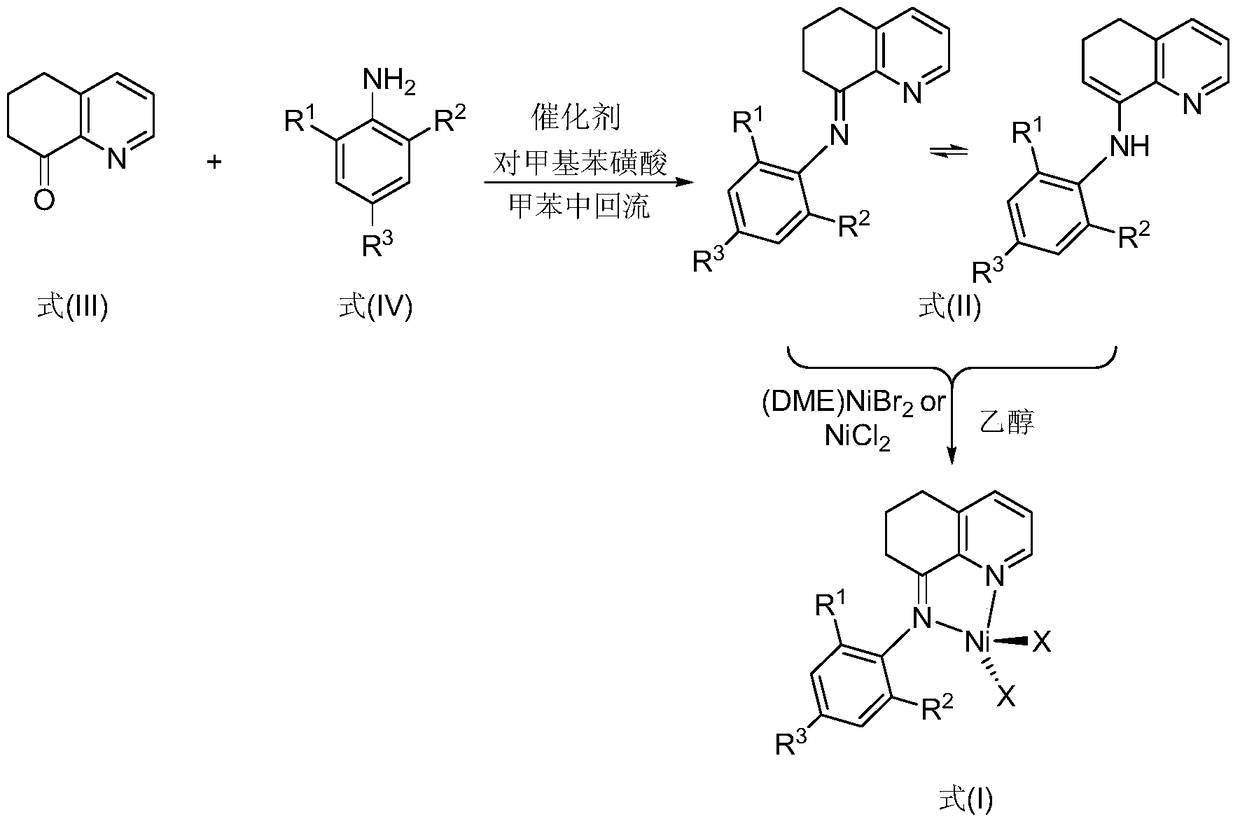
[0068] Example 1. Preparation of 8-(2,6-dicyclohexylaniline)-5,6,7-trihydroquinoline (L1) and 8-(2,6-dicyclohexylaniline)-5 shown in formula II ,Isomers of 6-dihydroquinoline (L1')
[0069] The hydroquinolinone compound represented by formula III was purchased from Shijiazhuang Likang Pharmaceutical Technology Co., Ltd., and the compound represented by formula IV was prepared according to the literature (Dokl. Phys. Chem., 2000, 374, 203-205). Add 43mg p-toluenesulfonic acid as a catalyst to compound hydroquinolinone (0.37g, 2.5mmol) shown in formula III and compound 2,6-dicyclohexylaniline (0.51g, 2.0mmol) shown in formula IV, and reflux in toluene After 4 hours, after concentration, the residue was separated by silica gel column chromatography, rinsed with petroleum ether / triethylamine (volume ratio 250:1), to obtain isomers of yellow oil (L1:L1'=36: 64) 0.25 g, yield 32%.
[0070] The NMR, IR and other structural confirmation data of the compound are as follows:
[0071]...
Embodiment 2
[0075] Example 2, preparation of 8-(2,6-dicyclopentylaniline)-5,6,7-trihydroquinoline (L2) and 8-(2,6-dicyclopentylaniline) shown in formula II Isomers of -5,6-dihydroquinoline (L2')
[0076] The compound hydroquinolinone (0.37g, 2.5mmol) shown in formula III and the compound 2,6-dicyclopentylaniline (0.46g, 2.0mmol) shown in formula IV were added into 43mg p-toluenesulfonic acid as catalyst, in toluene Reflux for 4 hours, after concentration, the residue was separated by silica gel column chromatography, rinsed with petroleum ether / triethylamine (volume ratio 250:1), to obtain isomers of yellow oil (L2:L2'=17 :83) 0.27g, the productive rate is 38%.
[0077] The NMR, IR and other structural confirmation data of the compound are as follows:
[0078] 1 H NMR (400MHz, CDCl 3 ,TMS): δ8.74(d,J=4.0Hz,1H,L2-Py H),8.41(d,J=4.8Hz,L2'-Py H),7.57(d,J=7.6Hz,1H, L2-Py H), 7.43 (d, J=7.6Hz, L2'-Py H), 7.44–7.28 (m, 1H, L2-Py H and L2'-Py H), 7.19 (s, L2'-Ar H), 7.13 (d, J=7.6Hz, 2H, L...
Embodiment 3
[0082] Example 3, preparation of 8-(2,4-dimethyl-6-cyclohexylaniline)-5,6,7-trihydroquinoline (L3) and 8-(2,4-dimethyl Isomers of -6-cyclohexylaniline)-5,6-dihydroquinoline (L3')
[0083] Compound hydroquinolinone (0.37g, 2.5mmol) shown in formula III and compound 2,4-dimethyl-6-cyclohexylaniline (0.41g, 2.0mmol) shown in formula IV were added to 43mg p-toluenesulfonic acid as Catalyst, refluxed 4 hours in toluene, after concentrating, the residue was separated by silica gel column chromatography, washed with sherwood oil / triethylamine (volume ratio 250:1), to obtain the isomers (L3: L3'=60:40) 0.20 g, yield 30%.
[0084] The NMR, IR and other structural confirmation data of the compound are as follows:
[0085] 1 H NMR (400MHz, CDCl 3 ,TMS):δ8.74(d,J=4.4Hz,1H,L3-Py H),8.42(d,J=4.8Hz,L3'-Py H),7.56(d,J=7.6Hz,1H, L3-Py H), 7.44 (d, J=7.2Hz, L3'-Py H), 7.30–7.26 (m, 1H, L3-Py H), 7.11–7.08 (m, L3'-Py H), 6.97 (s,L3'-Ar H),6.92(s,2H,L3-Ar H),6.84(s,L3'-Ar H),6.55(s,L3'-NH),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com