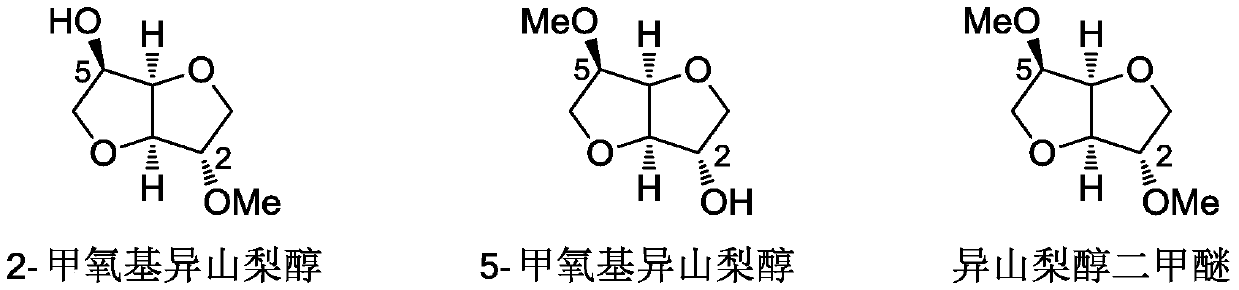
A method for synthesizing 1,4:3,6-dianhydrohexitol methyl ether
A technology of dianhydrohexitol and anhydrohexose, applied in the field of synthesizing 1,4:3,6-dianhydrohexitol methyl ether, which can solve the problem of low total yield of isosorbide methyl ether and reduction of acidic catalyst Activity, inhibiting the degree of hydroxyl etherification, etc., to achieve the effect of avoiding CO2 generation, avoiding corrosion problems, and improving the degree of hydroxyl etherification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
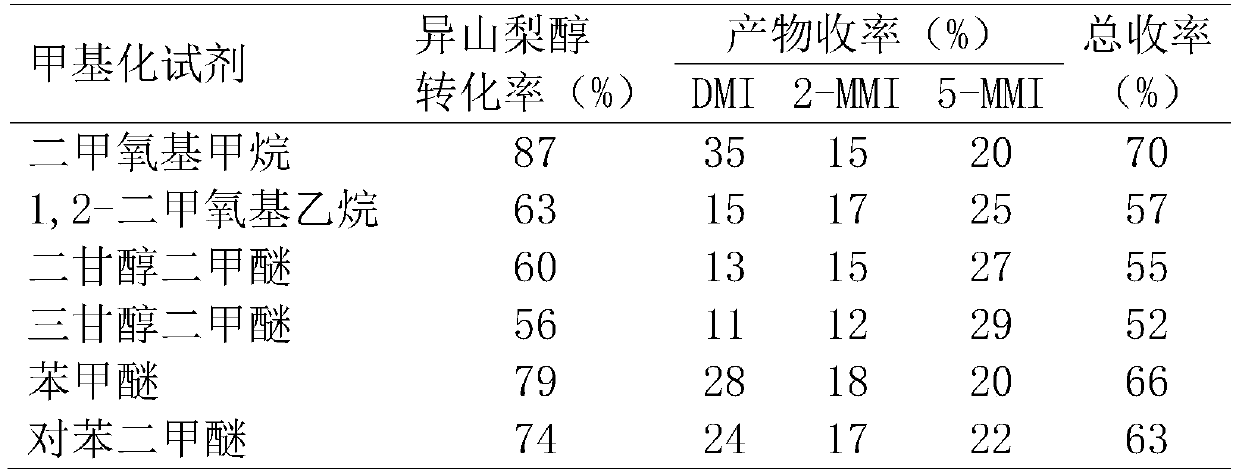
[0030] Put isosorbide, methyl ether compound and acidic catalyst Amberlyst-35 into a stainless steel reactor, seal the reactor, and stir magnetically at 130°C for 15 hours. Wherein, the molar ratio of methylating reagent and isosorbide is 50:1, and the mass ratio of catalyst Amberlyst-35 and isosorbide is 0.5:1. After the reactor was cooled to room temperature, the reaction solution was analyzed by gas chromatography. The conversion rate of isosorbide and the yield of methylated products were calculated according to the internal standard method of gas chromatography, expressed in mole percent (mol%).
[0031] The reaction results are shown in Table 1.
[0032] Table 1: Results of the reaction of isosorbide with methyl ether compounds.
[0033]
[0034] Wherein, the methylated products of isosorbide are 2-methoxyisosorbide (2-MMI), 5-methoxyisosorbide (5-MMI) and dimethyl isosorbide (DMI). Because the selected methyl ether compound has an active methyl group, it can be us...
Embodiment 2
[0036] Put isosorbide, 1,2-dimethoxyethane and strong acid ion exchange resin into a stainless steel reactor, seal the reactor, and react with magnetic stirring at 150°C for 3 hours. Wherein, the molar ratio of 1,2-dimethoxyethane to isosorbide is 10:1, and the mass ratio of strong acid ion exchange resin to isosorbide is 0.3:1. After the reactor was cooled to room temperature, the reaction solution was analyzed by gas chromatography. The conversion rate of isosorbide and the yield of methylated products were calculated according to the internal standard method of gas chromatography, expressed in mole percent (mol%).
[0037] The reaction results are shown in Table 2.
[0038] Table 2: The results of the reaction between isosorbide and 1,2-dimethoxyethane catalyzed by different strong acid ion exchange resins.
[0039]
[0040] The strong acid ion exchange resin has a good catalytic effect on the methylation reaction of isosorbide and 1,2-dimethoxyethane. When the conversi...
Embodiment 3
[0042] Put isosorbide, 1,2-dimethoxyethane and heteropoly acid into a stainless steel reactor, seal the reactor, and react with magnetic stirring at 150°C for 5 hours. Wherein, the molar ratio of 1,2-dimethoxyethane to isosorbide is 20:1, and the mass ratio of heteropoly acid to isosorbide is 0.2:1. After the reactor was cooled to room temperature, the reaction solution was analyzed by gas chromatography. The conversion rate of isosorbide and the yield of methylated products were calculated according to the internal standard method of gas chromatography, expressed in mole percent (mol%).
[0043] The reaction results are shown in Table 3.
[0044] Table 3: The reaction results of heteropolyacid-catalyzed isosorbide and 1,2-dimethoxyethane.
[0045]
[0046] Phosphotungsten and silicotungstic heteropolyacids have better catalytic effects on the methylation of isosorbide and 1,2-dimethoxyethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com