Application of asymmetric aryl disulfide compounds serving as virus 3C protease inhibitors in preparation of antiviral drugs
An antiviral drug and disulfide technology, applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of no myocarditis virus 3C protease drug and single compound structure type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Synthesis of unsymmetrical aromatic disulfide compounds
[0037]
[0038] The reaction equation is shown above.
[0039] Add 0.50g (5mmol) of 3-mercapto-[1,2,4]-triazole-to 0.94g (5mmol) of the ether solution of o-nitrophenylsulfanyl chloride, react at room temperature for 3 hours, filter, and anhydrous After washing with ether, the target compound was obtained, and the crude product was passed through the column to obtain 1.13 g of yellow o-nitrophenyldithio-1H-[1,2,4]-triazole (compound represented by formula 1, yield 89%).
[0040] The compound shown in formula 2-11 was synthesized basically according to the same method.
[0041] The physical and chemical characterization data of the compound shown in formula 1-11 is shown in Table 1, 1 HNMR, high-resolution mass spectrometry data are shown in Table 2.
[0042] Table 1 Physicochemical parameters of unsymmetrical aromatic disulfide compounds
[0043]
[0044]
[0045] Table 2 Asymmetric arom...
Embodiment 2
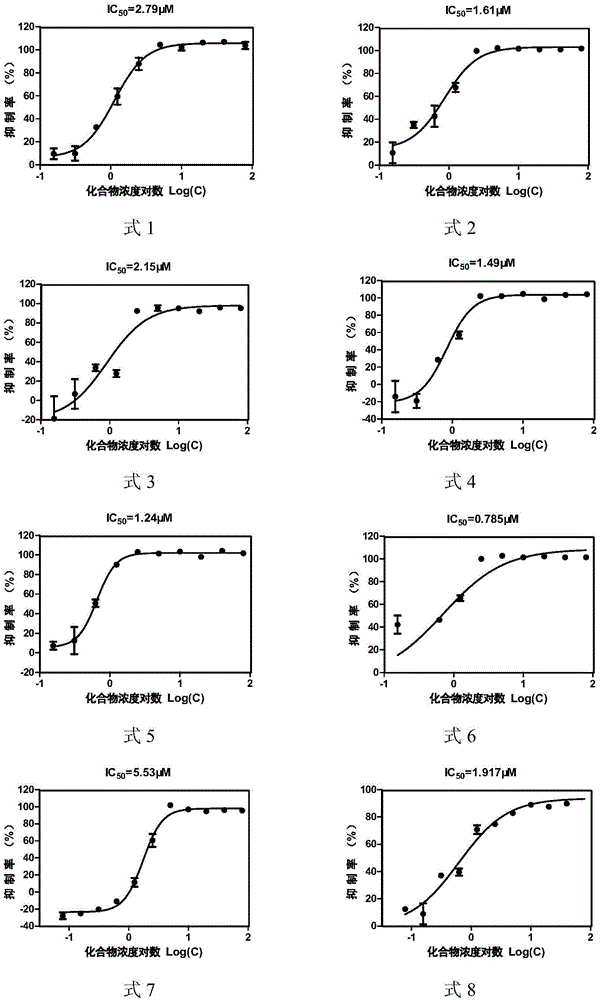
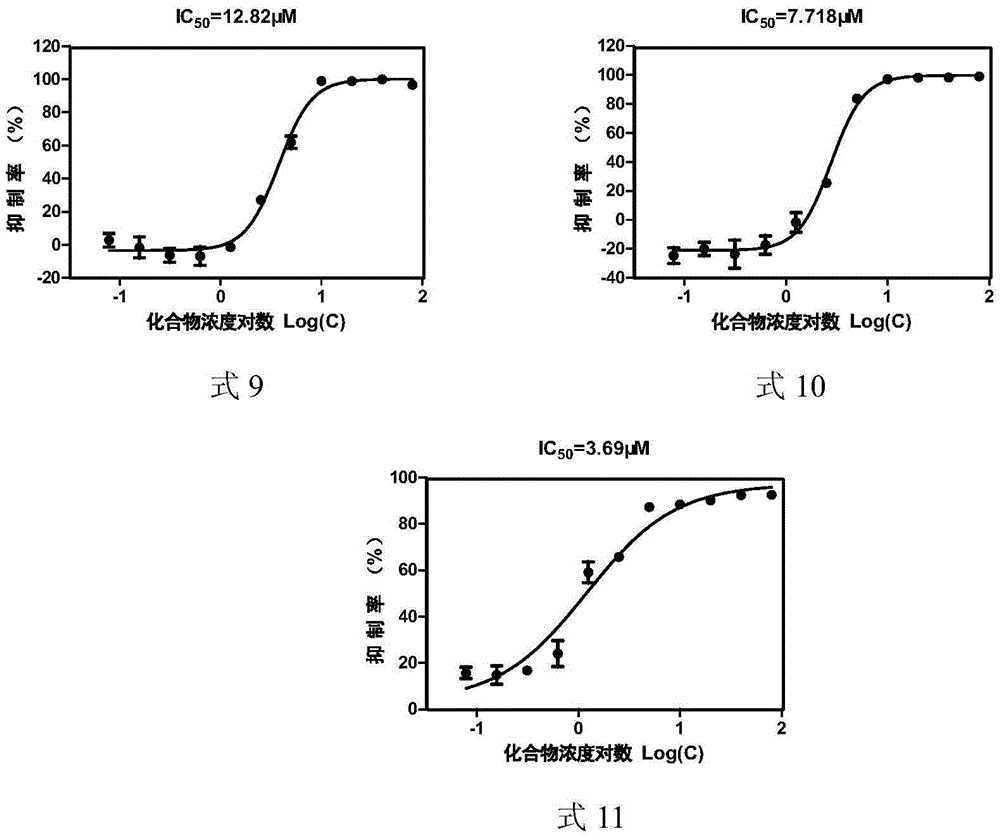
[0048] Example 2: Determination of CVB33C protease inhibitory activity of asymmetric aromatic disulfide compounds
[0049] The total volume of each well of the test model is 50 μL, including 3C protease solution (final concentration 2.3 μM), substrate solution (final concentration 25 μM) and PBS-BSA buffer (2×PBS buffer with additional 2 mg / mL BSA and 5.85 mg / mL NaCl ), each compound had a two-fold concentration gradient, and the final concentration of asymmetric aromatic disulfide compounds was 0-200 μM; a negative control (without CVB33C protease) and a blank group (DMSO) were set at the same time, and a positive control rufentravir was also set Group. Three parallel sets were set up for each group. After mixing and incubating at 37°C for 60 minutes, the fluorescence value was read with a SpectramaxGEMINIxps fluorescence microplate reader. The excitation wavelength was 340nm and the emission wavelength was 490nm. The value of each hole was subtracted from the blank group to...
Embodiment 3
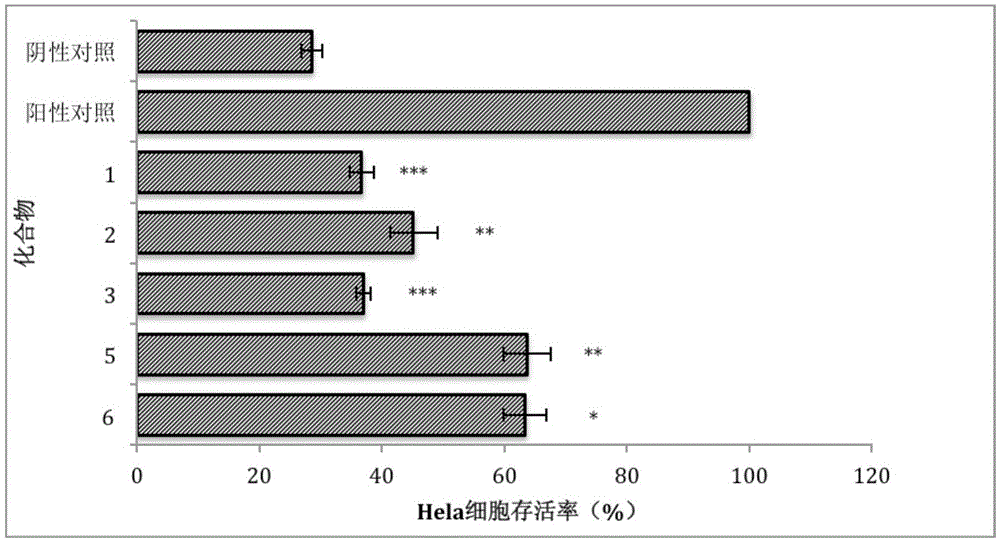
[0052] Embodiment 3: Test of the cytotoxicity of asymmetric aromatic disulfide compounds to Hela cells
[0053] The total volume of each well of the test model was 100 μL, and inoculated with human cervical cancer cell Hela cells (final concentration 5×10 5 cells / mL), at 37°C, 5% CO 2 After culturing under the conditions for 24 hours, different concentrations of the tested compounds were added, and the incubation was continued for 16 hours, then 10 μL CCK-8 solution was added, and the absorbance at a wavelength of 450 nm was detected after 4 hours. In this model, a model group (DMSO) and a blank group (without DMSO) were set at the same time, and each group was set with 3 parallels.
[0054] The results are shown in Table 3. It can be seen from Table 3 that each compound has 50 There was no cytotoxicity to Hela cells within the dose range.
[0055] Inhibitory activity and cytotoxicity of asymmetric aromatic disulfide compounds shown in table 3 formula 1-11 to CVB33C proteas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com