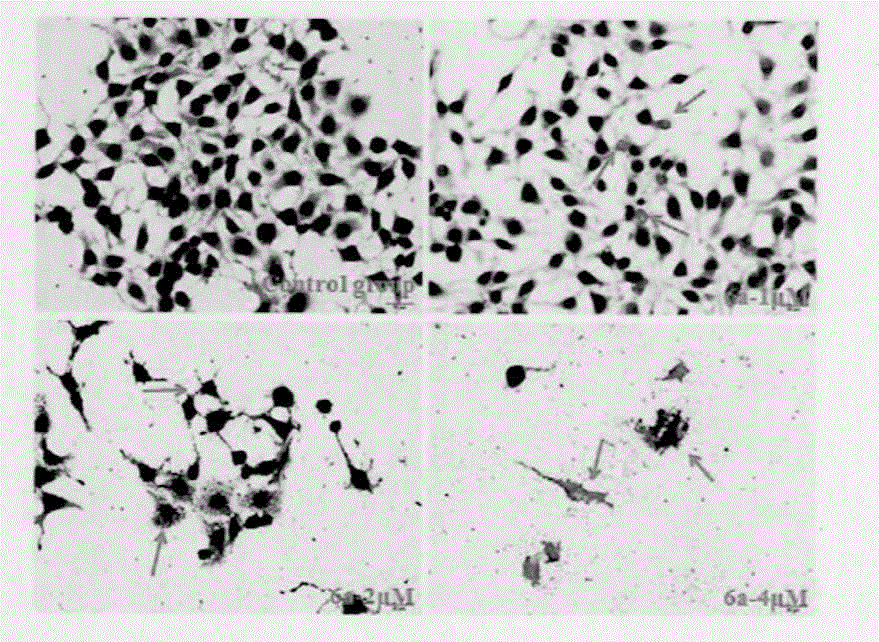
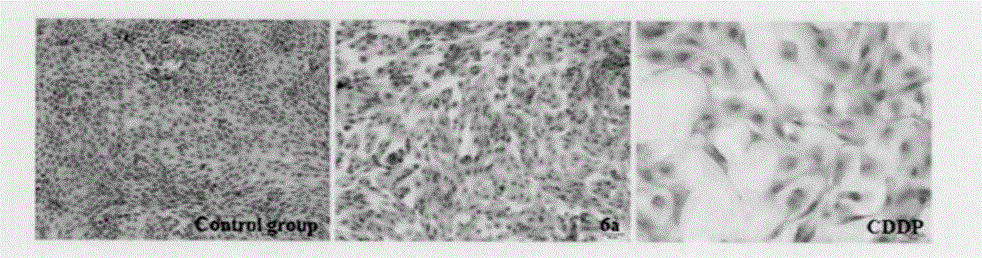
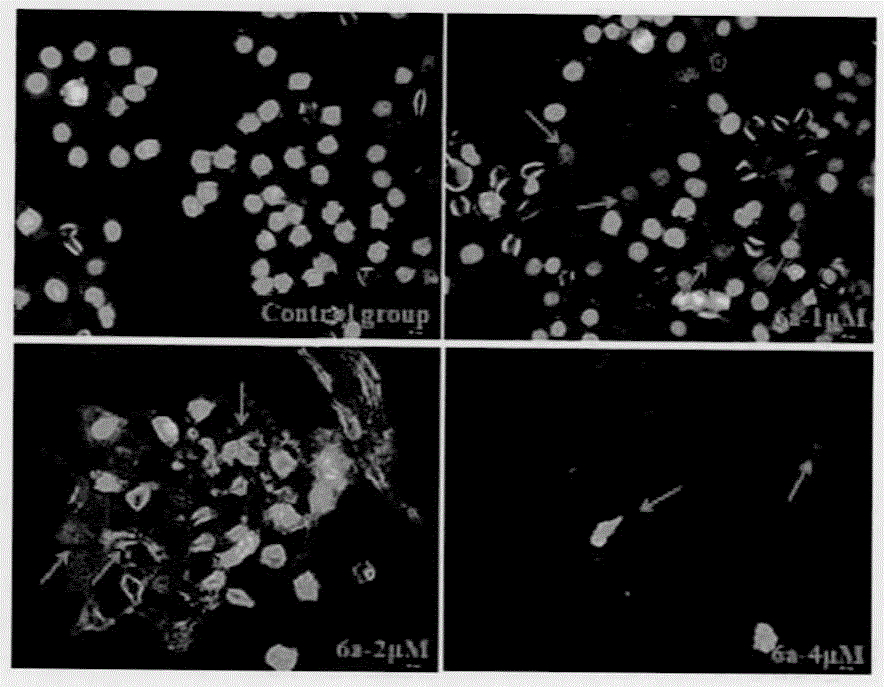
Preparation method and application of antitumor drug X-TOA
A technology of X-TOA and compounds, applied in the general formula of X-TOA and its synthesis and application fields, can solve problems such as not yet developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Preparation of Compound 2-Aomethyl-3,5,6-Trimethylpyrazine
[0085] According to "Optimization of the synthesis process of intermediate 2-Aomethyl-3,5,6-trimethylpyrazine". Xu Kuo, Wang Penglong, Han Qiujun, etc. Anhui Medicine, 2013, 17(9): 1467- 1470" method. Weigh 20.00g (0.15mol) anhydrous ligustrazine, 23.54g NBSN-bromosuccinimide (0.15mol, finely ground before use) into a 250mL three-necked flask, 100mLCCl 4 As the reaction solvent, 4 85W incandescent lamps were irradiated, and the reaction was refluxed at 95°C for 1 hour. TLC [V (petroleum ether): V (acetone) = 3:1] detected that the reaction was basically complete; after cooling, it was filtered, the filtrate was collected, and the solvent was recovered under reduced pressure to obtain a purple-red viscous liquid (content is 60%). HRMS(ESI)m / z: 216.00135[M+H] + , Theoretical calculation: C 8 H 11 BrN 2 : 216.00851.
Embodiment 2
[0086] Example 2 Preparation of compound G-TOA
[0087] Weigh 66.57mg (0.38mmol) Boc-L-glycine, 95.50mg (0.50mmol) EDCI, 3.05mg (0.025mmol) DMAP and 147.75mg (0.25mmol) TOA in a 50mL single-neck flask, and add an appropriate amount of dichloride Methane dissolved it and stirred overnight at room temperature. TLC [V(dichloromethane): V(methanol)=20:1] detects the reaction progress, the reaction solution is extracted with dichloromethane, the organic layer is collected, an appropriate amount of anhydrous sodium sulfate is removed from the water, evaporated under reduced pressure, and separated by silica gel column [V(dichloromethane):V(methanol)=20:1] to obtain a white solid; dissolve it in ethyl acetate containing 3M HCl, stir in an ice-salt bath for 1 h; evaporate the reaction solution under reduced pressure and use appropriate amount of ethyl acetate Reconstitute, adjust the pH to neutral with saturated sodium bicarbonate solution, collect the organic layer, remove water with a...
Embodiment 3
[0088] Example 3 Preparation of Compound A-TOA
[0089] Weigh 71.90mg (0.38mmol) Boc-L-alanine, 95.50mg (0.50mmol) EDCI, 3.05mg (0.025mmol) DMAP and 147.75mg (0.25mmol) TOA in a 50mL single-necked flask, and add an appropriate amount of Dissolve with dichloromethane and stir overnight at room temperature. According to the operation of Example 2, a white solid with a melting point of 89.7-92.1°C was obtained, (c1.0, methanol), yield: 56%. 1 H-NMR(CDCl 3 )δ(ppm): 0.54, 0.89, 1.11(s, each, 3H, 3×-C H 3 , The methyl group of oleanolic acid), 0.87 (brs, 6H, 2×-C H 3 , The methyl group of oleanolic acid), 0.91 (brs, 6H, 2×-C H 3 , The methyl group of oleanolic acid), 2.49, 2.51, 2.55 (s, each, 3H, 3×-C H 3 , The methyl group of ligustrazine), 2.74 (brs, 2H, -N H 2 ), 2.86(m, 1H, -CH=CC H -), 3.58(s, 1H, -NH 2 C H -), 4.53(m, 1H, -OCOC H -), 5.12, 5.19 (d, each, J=10Hz, 1H, -OC H 2 -), 5.24 (brs, 1H, -C H =C-), 1.00-2.50 (25H, methyl of alanine, methine and methylene of oleanolic acid);...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com