A kind of modified polyvinyl carbazole polymer luminescent material and preparation method thereof
A technology of polyvinyl carbazole and vinyl carbazole, which is applied in the field of organic light-emitting materials, can solve the problem of low polymer chain molecular weight, which cannot solve the material fluorescence self-quenching excimer, and the method conditions are harsh and other problems, to achieve the effect of high fluorescence intensity, good solubility, and easy post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
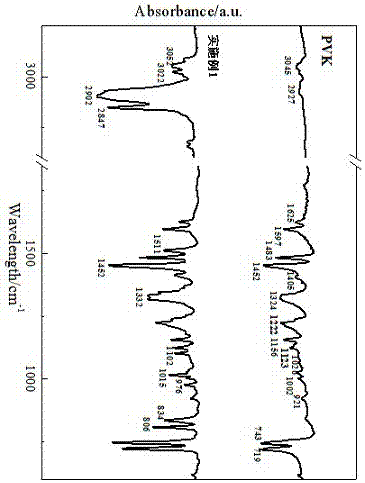
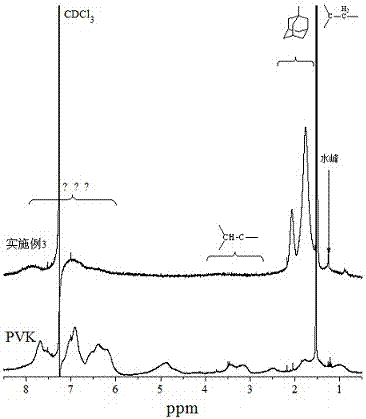
Image
Examples
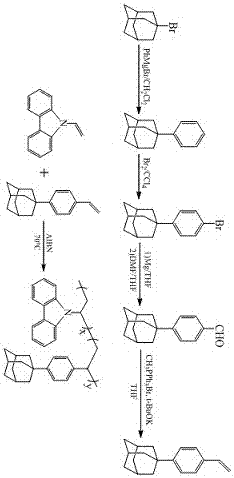
Embodiment 1
[0031] (1) Synthesis of 1-phenyladamantane
[0032] Mg (7.22g, 0.297mol) and anhydrous ether (20ml) were added to a 150ml three-necked flask, activated with a small amount of iodine, and anhydrous bromobenzene (28.5g, 0.182 g) diluted with 100ml anhydrous ether was added dropwise under nitrogen atmosphere. mol) mixture was added and stirred at room temperature for 1 h. After the reaction, the liquid phase was carefully transferred to another flask to remove unreacted Mg, and the excess ether solvent was distilled off under reduced pressure. Then add dissolved in 140 ml dry CH under nitrogen flow 2 Cl 2 1-Bromoadamantane (12.9g, 0.060mol) in the mixture was refluxed for 24h. Then cooled to room temperature, slowly poured into 2M hydrochloric acid solution, separated, and the aqueous phase was washed with CH 2 Cl 2 Extracted three times, combined the organic phases, washed the organic layer with water, and then washed it with anhydrous MgSO. 4 Drying and distillation under...
Embodiment 2
[0045] (1) Synthesis of 1-phenyladamantane
[0046] Mg (5.30g, 0.2219mol) and anhydrous ether (15ml) were added to a 150ml three-necked flask, activated with a small amount of iodine, and anhydrous bromobenzene (28.5g, 0.182 g) diluted with eternal 100ml anhydrous ether was added dropwise under nitrogen atmosphere. mol) mixture was added and stirred at room temperature for 2h. After the reaction, the liquid phase was carefully transferred to another flask to remove the remaining Mg, and the excess ether solvent was evaporated under reduced pressure. Then add dissolved in 140 ml dry CH under nitrogen flow 2 Cl 2 1-Bromoadamantane (12.9g, 0.060mol) in the mixture was refluxed for 24h. Then cooled to room temperature, slowly poured into 2M hydrochloric acid solution, separated, and the aqueous phase was washed with CH 2 Cl 2 Extracted three times, combined the organic phases, washed the organic layer with water, and then washed it with anhydrous MgSO. 4 Drying and distillat...
Embodiment 3
[0056] (1) Synthesis of 1-phenyladamantane
[0057] Add Mg (8.85g, 0.365mol) and anhydrous ether (25ml) to a 150ml three-necked flask, activate with a small amount of iodine, and then add dropwise anhydrous bromobenzene ( 28.5g, 0.182mol) mixed solution, after adding, it was stirred at room temperature for 3h. After the reaction was completed, the liquid phase was carefully transferred to another flask to remove residual Mg, and the excess ether solvent was distilled off under vacuum. Then add dissolved in 140 ml dry CH under nitrogen flow 2 Cl 2 1-Bromoadamantane (12.9g, 0.060mol) in the mixture was refluxed for 24h. Then cooled to room temperature, slowly poured into 2M hydrochloric acid solution, separated, and the aqueous phase was washed with CH 2 Cl 2 Extracted three times, combined the organic phases, washed the organic layer with water, and then washed it with anhydrous MgSO. 4 Dry and distilled under reduced pressure to obtain a pale yellow solid, which was pass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com