Coumarin-based light-CO2/N2 double stimuli-responsive surfactant, and preparation method thereof
A dual-stimuli-response, surfactant technology, applied in chemical instruments and methods, chemical/physical processes, organic chemistry, etc., can solve problems such as easy separation of surfactants that are difficult to achieve reversible regulation, increased chemical substance emissions and pollution, etc. Achieving a mild method, easy separation, and inexpensive environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
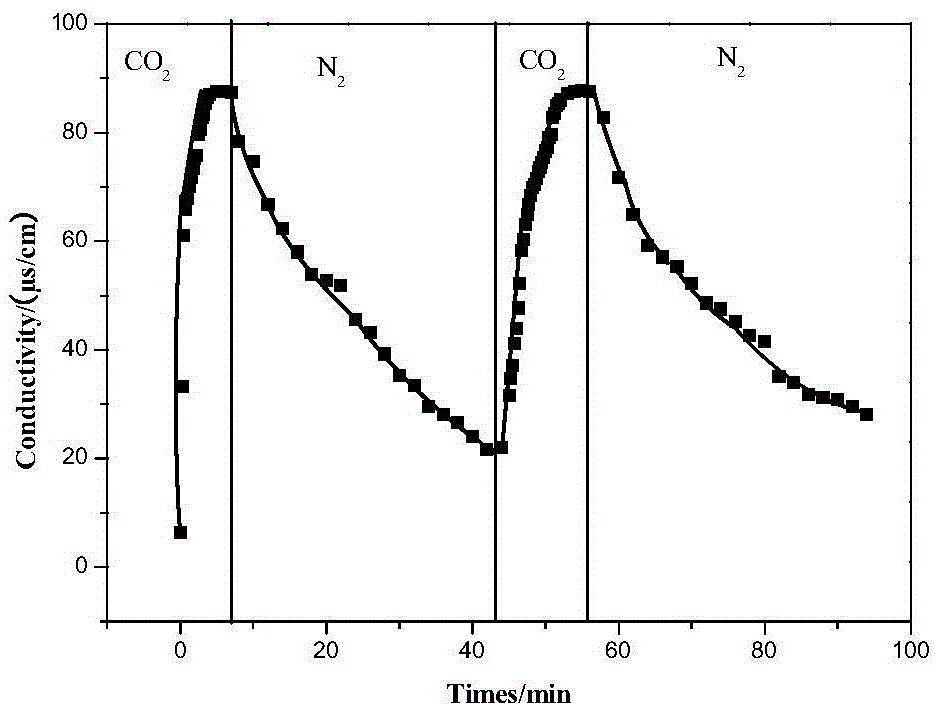
Examples
Embodiment 1
[0028] (1) Synthesis of coumarinyl bromodecane
[0029] Add 7-hydroxycoumarin (2g), 1,10-dibromodecane (14.8g) and anhydrous potassium carbonate (3.5g) into a 250ml round bottom flask, and use acetone dried over anhydrous magnesium sulfate as solvent , Reflux reaction at 55°C for more than 24h. After cooling, the solvent was removed by rotary evaporation. The product was dissolved in 100ml of deionized water, extracted twice with dichloromethane, the organic layer was washed three times with deionized water, dried over anhydrous magnesium sulfate, and dichloromethane was removed by rotary evaporation to obtain a yellow-green viscous liquid. Pass the crude product through the column, the eluent is petroleum ether:ethyl acetate=10:1, and the white solid is 1-coumarinyl-10-bromodecane. The resulting product was characterized by mass spectrometry (see figure 2 ),Depend on figure 2 It can be seen that the synthesized product is the target product, that is, 1-coumarinyl-10...
Embodiment 2
[0035] (1) Synthesis of coumarinyl bromohexane
[0036] Add 7-hydroxycoumarin (2g), 1,6-dibromohexane (13.1g) and anhydrous potassium carbonate (3.5g) into a 250ml round-bottomed flask, and use acetone dried over anhydrous magnesium sulfate as a solvent , Reflux reaction at 60°C for more than 24h. After cooling, the solvent was removed by rotary evaporation. The product was dissolved in 100ml of deionized water, extracted twice with dichloromethane, the organic layer was washed three times with deionized water, dried over anhydrous magnesium sulfate, and dichloromethane was removed by rotary evaporation to obtain a yellow-green viscous liquid. Pass the crude product through the column, the eluent is petroleum ether:ethyl acetate=10:1, and the white solid is 1-coumarinyl-6-bromohexane.
[0037](2) Synthesis of coumarin-based tertiary amines
[0038] 1-coumarinyl-6-bromohexane was reacted with 10 equivalents of dimethylamine hydrochloride, excess triethylamine was added to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com