Novel sorafenib TsOH crystal form as well as preparation method and application thereof
A technology of sorafenib p-toluenesulfonate and p-toluenesulfonic acid, which is applied in the field of medicine and can solve problems such as the possibility of increasing solvent residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
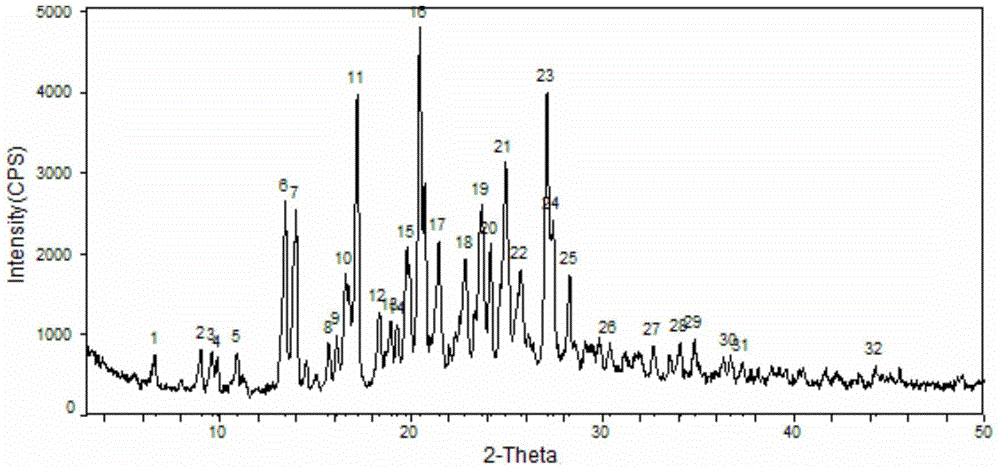
Image
Examples
Embodiment 1
[0059] Preparation of the formula (II) compound of sorafenib p-toluenesulfonate monohydrate type E crystal:
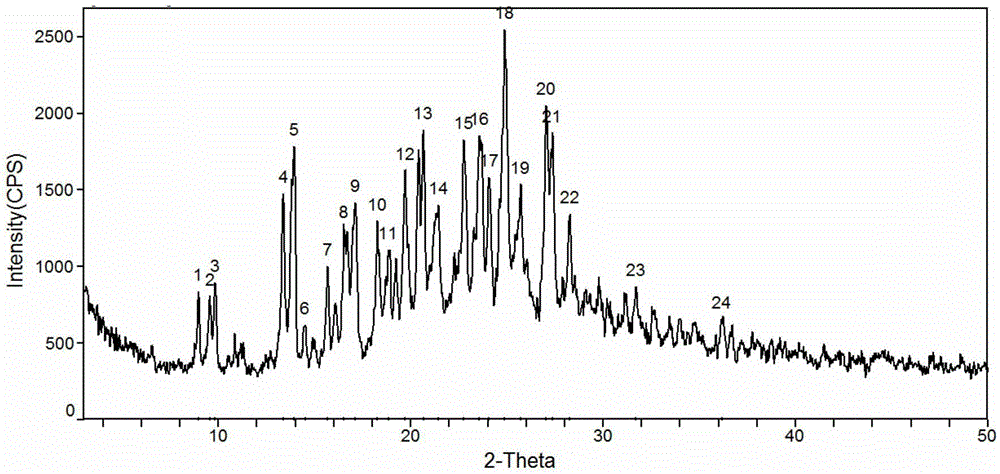
[0060] Dissolve 2g of sorafenib free base in 15ml of 1,4-dioxane and 5ml of deionized water, stir, heat up to 78°C, add 1g of 1,4-p-toluenesulfonic acid monohydrate after the system dissolves quickly Dioxane solution 3ml, keep stirring for 30 minutes. The system was naturally cooled to room temperature, then cooled to -5-0°C with freezing liquid, and continued to stir and crystallize for 20 hours. Suction filtration, wash with 1,4-dioxane, collect filter cake, blow dry at 50°C for 72 hours, measure moisture after drying to 3.0, and obtain 2.4g of sorafenib p-toluenesulfonate monohydrate, yield 88%, purity 98.79%, melting point: 127.3~134.8℃, X-ray powder diffraction pattern as figure 1 As shown, the TGA graph is shown as figure 2 shown.
Embodiment 2
[0062] Preparation of the formula (II) compound of sorafenib p-toluenesulfonate monohydrate type E crystal:
[0063] Dissolve 2.2g of sorafenib free base in 44ml of 1,4-dioxane and 4.4ml of deionized water, stir, heat up to 76°C, and add 1g of p-toluenesulfonic acid monohydrate after the system dissolves rapidly. , 4 dioxane solution 3ml, continue to stir for 30 minutes. Naturally cool down to room temperature, then cool down to -5-0°C, and continue to stir and crystallize for 25 hours. Filter with suction, wash the filter cake with 1,4-dioxane, and collect the filter cake. Air-drying at 60°C for 16 hours, the measured water content after drying was 3.22, and 2.5 g of sorafenib p-toluenesulfonate monohydrate was obtained with a yield of 84%. Purity: 98.59%, melting point: 126.0~135.1℃.
Embodiment 3
[0065] Preparation of the formula (II) compound of sorafenib p-toluenesulfonate monohydrate type E crystal:
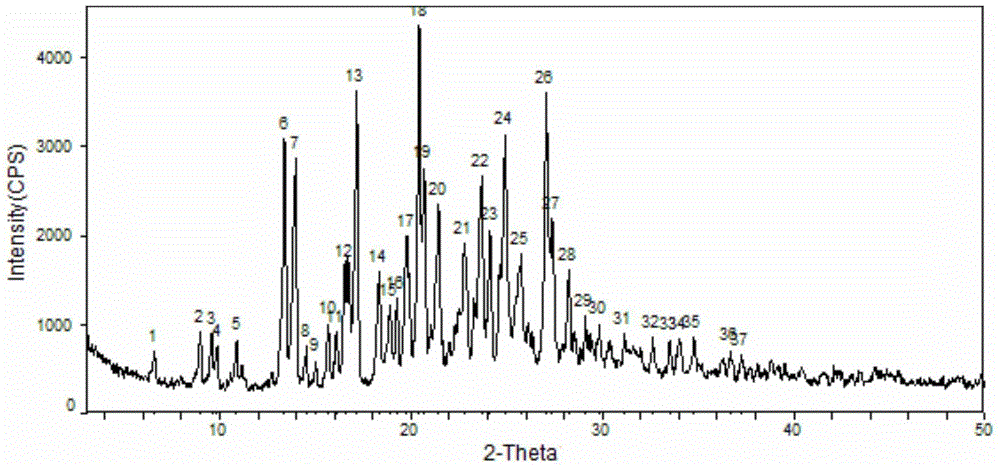
[0066] Dissolve 1g of sorafenib free base in 8ml of 1,4-dioxane and 2ml of deionized water, stir, heat up to 76°C, add 0.4g of p-toluenesulfonic acid monohydrate 1, 4 dioxane solution 2ml, continue to stir for 1 hour. Cool down to room temperature (15-18°C) naturally, and stir and crystallize for 17 hours. Filter with suction, wash the filter cake with 1,4-dioxane, and collect the filter cake. Air-dried at 60°C for 6 hours, and measured moisture content after drying was 3.20 to obtain 0.9 g of sorafenib p-toluenesulfonate monohydrate with a yield of 90%. Purity 98.86%, melting point: 133.2-136.9°C, X-ray powder diffraction pattern is as follows image 3 as shown, 1 H-NMR detection results such as Figure 7 Shown (DMSO-d 6 ,400MHzδppm=2.29(s,1.5H),2.83(s,3H),5.79(br,2.5H),7.17~7.21(m,3H),7.25~7.27(m,1H),7.58~7.70(m, 6H), 8.14(d,1H), 8.57(d,1H), 9.03(d,1H), 9.27(s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com