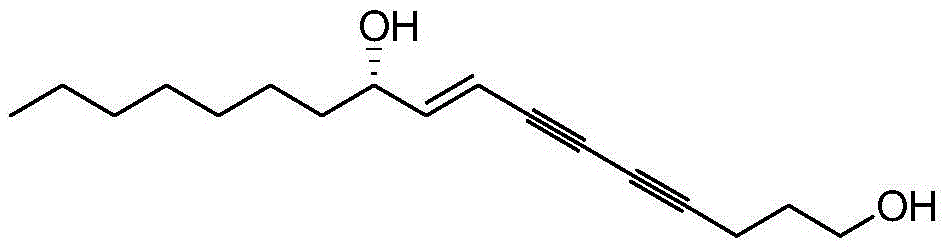
Polyacetylene type cowbane extract (S)-Virol C and synthetic method thereof
A technology of extract and polyyne, which is applied in the field of asymmetric synthesis of hemlock extract-VirolC, can solve the problems of difficult extraction and synthesis methods, decomposition and deterioration, and achieve cost reduction, simplification of reaction operation and synthesis route, and preparation process Efficient and environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The following examples further illustrate the content of the present invention, but should not be construed as limiting the present invention.
[0023] 1. Synthesis of (S)-VirolC(I):
[0024] Under nitrogen protection, in a 10mL Shrek tube equipped with a magnetic stirrer, add cuprous iodide (57mg, 0.3mmol), tetrakistriphenylphosphine palladium (86mg, 0.75mmol), (S, E)-1 -Bromo-1-ene-3-decanol (II) (1.5mmol) and 3mL of benzene, stirred evenly, reacted at room temperature for 3 minutes, then added 4,6-diyne-1-heptanol (III) (1.5 mmol) and triethylamine (2 mL, 15 mmol), react overnight at room temperature. The reaction was quenched by adding 3 mL of saturated ammonium chloride solution. The aqueous phase was extracted with ether, the obtained organic phase was dried with anhydrous sodium sulfate, and the crude product was obtained by distillation under reduced pressure. Finally, it was purified by silica gel column chromatography (petroleum ether / ethyl acetate=10 / 1) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com