A gelatin-free varicella vaccine freeze-dried preparation and a preparing method thereof
A technology for freeze-dried preparations and vaccines, which can be used in freeze-dried transportation, antiviral agents, and pharmaceutical formulations, and can solve problems such as insufficient establishment of freeze-dried scaffolds, easy allergic reactions, and potential safety hazards of animal-derived viruses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] The preparation of the vaccine stabilizer of the present invention can be carried out according to pharmaceutically acceptable conventional methods. For example, each component in the vaccine stabilizer is mixed according to its specific acceptable concentration, and the pH value is adjusted, so that the pH value of the vaccine preparation is maintained at 7 after reconstitution of the lyophilized preparation.
[0065] buffer
[0066] The buffer that can be used in the lyophilized formulation of the varicella vaccine of the present invention is not particularly limited, and can be any buffer that makes the lyophilized formulation of the varicella vaccine of the present invention have a pH of 7.0 after reconstitution. Preferably, the buffer that can be used in the present invention is PBS (phosphate) buffer. Contains sodium chloride, potassium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate, dipotassium hydrogen phosphate, potassium dihydrogen phospha...
Embodiment 1
[0095] Embodiment 1 No gelatin stabilizer to the protective effect of vaccine stock solution
[0096] 1.1 Relevant quality indicators of vaccine stock solution
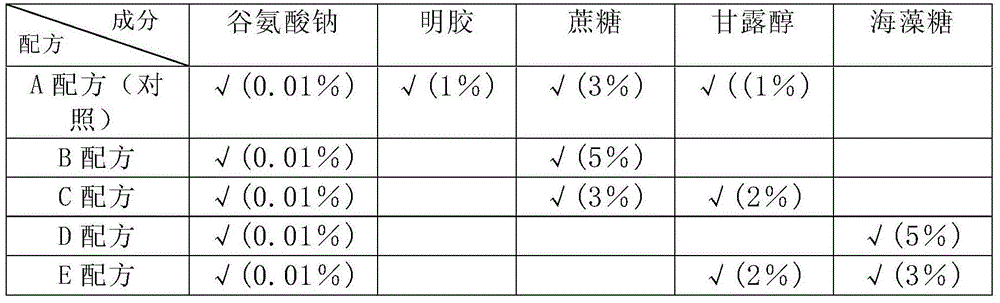
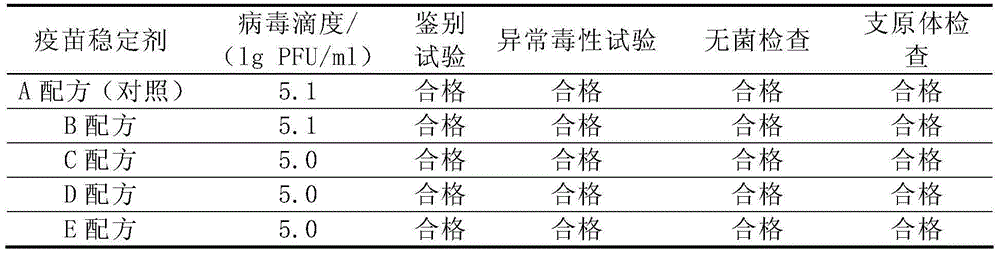
[0097] Table 2 shows that the virus titer of the stock solution of varicella live attenuated vaccine prepared with 4 kinds of gelatin-free stabilizers (B, C, D, E formula) is basically the same as that of the vaccine stock solution prepared with gelatin stabilizer (A formula), both It meets the requirements of the regulations; the identification test, abnormal toxicity test, sterility test and mycoplasma test of the five vaccine stock solutions are all qualified. Studies have shown that 4 gelatin-free stabilizer formulations have protective effects on the stock solution of live attenuated varicella vaccine.
[0098] The test results of relevant quality indicators of 25 kinds of varicella live attenuated vaccine stock solutions
[0099]
[0100] 1.2 Virus stability test of vaccine stock solution
[0101] The vacc...
Embodiment 2
[0106] Embodiment 2 No gelatin stabilizer to the protective effect of vaccine finished product
[0107] 2.1 Relevant quality indicators of finished vaccine products
[0108] 2.1.1 Appearance
[0109] According to the "Registration Standards for Varicella Live Attenuated Vaccines", the inspection of 5 kinds of finished vaccines shows that ( figure 1 ), the appearance of five kinds of vaccines after freeze-drying was white loose body, smooth surface, no shrinkage, foaming and swelling. After adding sterilized water for injection, it dissolves rapidly, and no foreign matter appears. Except for the use of gelatin-containing stabilizer, vaccine A was pale yellow after dissolving, and the rest of the vaccines were slightly white after dissolving due to the use of no gelatin stabilizer.
[0110] 2.1.2 Short-term thermal stability of finished product
[0111] The thermal stability test results (Table 4) of short-term storage at room temperature show that the virus titers of the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com