Bi-functional catalyst for alkaline water system metal/air battery and preparation method thereof
A dual-function catalyst, air battery technology, applied in battery electrodes, fuel cell-type half-cells and primary battery-type half-cells, circuits, etc. problem, to achieve the effect of good conductivity, easy to scale up production, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
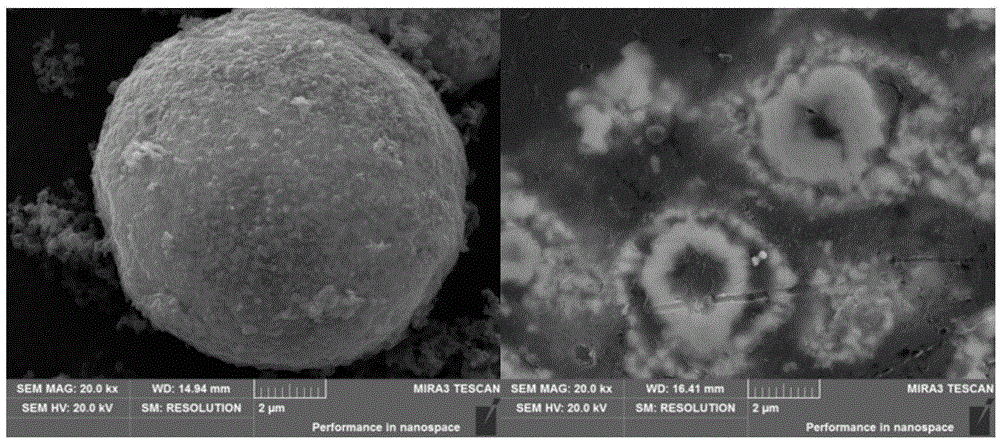
Image
Examples
Embodiment 1
[0042] Add 0.05mol urea to 90mL of 0.05M nickel nitrate solution, stir until it completely dissolves into a transparent solution, package it in a reaction kettle, set the reaction temperature to 120°C, and the reaction time to 4h. Wash several times, filter and dry at 50°C to obtain the reaction precursor; ultrasonically disperse 0.5g of the above reaction precursor in 500mL of deionized water, and slowly dissolve the dopamine hydrochloride Add it, adjust the pH value to 8, stir at room temperature for 12 hours, wash the obtained product with water and alcohol, and then dry it at 50°C; place the above-mentioned dried product in a tube furnace, set the heating rate to 5°C / min, and hold the temperature at 600°C, holding time for 2 hours, and cooling naturally to obtain N-doped carbon-coated composite metal element. The composite catalyst obtained by the present invention has a reduction potential of -0.28V (vs.Ag / AgCl) in a 0.1M oxygen-saturated KOH solution, and an oxygen preci...
Embodiment 2
[0044] Add 0.06mol urea to 90mL solution containing 0.07M nickel nitrate and 0.14M cobalt nitrate, stir until completely dissolved into a transparent solution, package it in a reaction kettle, set the reaction temperature to 120°C, and the reaction time to 6h. The final product was washed several times with water and alcohol, filtered and dried at 50°C to obtain the reaction precursor; the above 0.5g reaction precursor was ultrasonically dispersed in 500mL deionized water, and the mass ratio of the precursor to dopamine hydrochloride monomer was 1 : 1, slowly adding dopamine hydrochloride wherein, adjusting the pH value to 8.8, stirring at room temperature for 24h, washing the product with water and alcohol and then drying at 60°C; 5°C / min, the holding temperature is 600°C, the holding time is 2h, and natural cooling is performed to obtain the N-doped carbon-coated composite metal element. The reduction potential of the composite catalyst obtained in the invention is -0.20V (v...
Embodiment 3
[0046] Add 0.06mol urea to 90mL of 0.1M cobalt nitrate solution, stir until completely dissolved into a transparent solution, package it in a reaction kettle, set the reaction temperature to 110°C, and the reaction time to 6h. Wash several times, filter and dry at 50°C to obtain the reaction precursor; ultrasonically disperse 0.8 g of the above reaction precursor in 800 mL of deionized water, and slowly dissolve the dopamine hydrochloride Add it, adjust the pH value to 8.2, stir at room temperature for 18 hours, wash the product with water and alcohol, and then dry it at 70°C; put the dried product above in a tube furnace, set the heating rate to 3°C / min, and hold the temperature temperature at 620°C, holding time for 2 hours, and cooling naturally to obtain an N-doped carbon-coated composite metal element. The composite catalyst obtained by the present invention has a reduction potential of -0.30V (vs.Ag / AgCl) in a 0.1M oxygen-saturated KOH solution, and an oxygen precipitati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com