Preparation and application of fluorescent probe capable of being used for distinguishing cysteine/homocysteine and glutathione
A technology for homocysteine and cysteine, which is applied in the field of preparation of fluorescent probes for distinguishing between cysteine/homocysteine and glutathione, achieving fast response, good selectivity, and high resistance to glutathione. The effect of strong interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
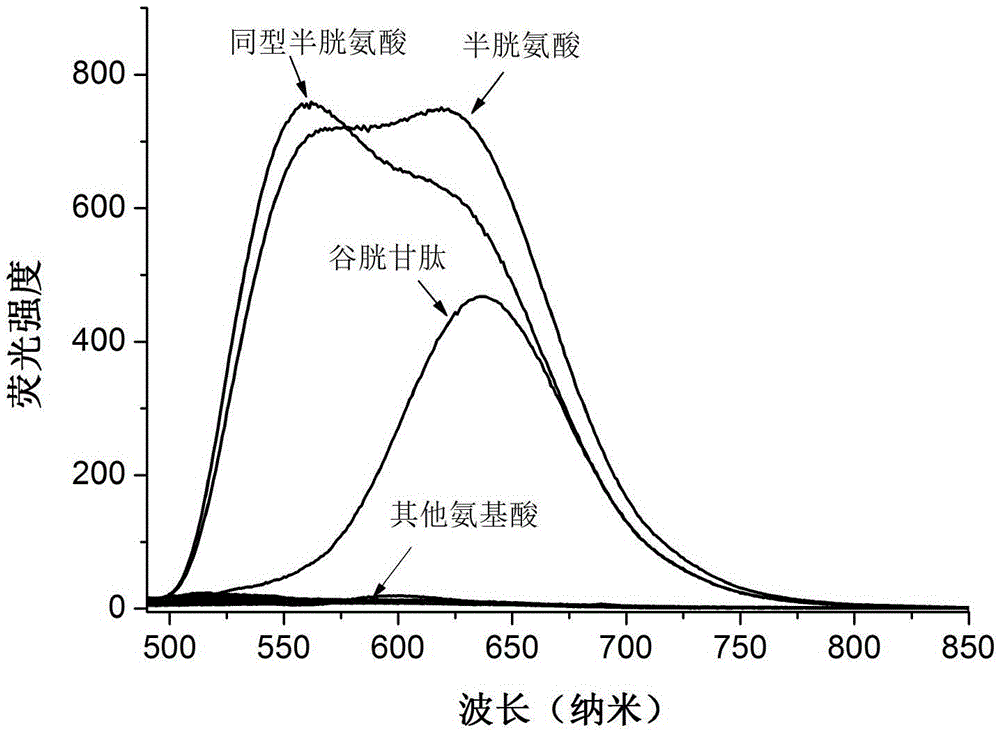
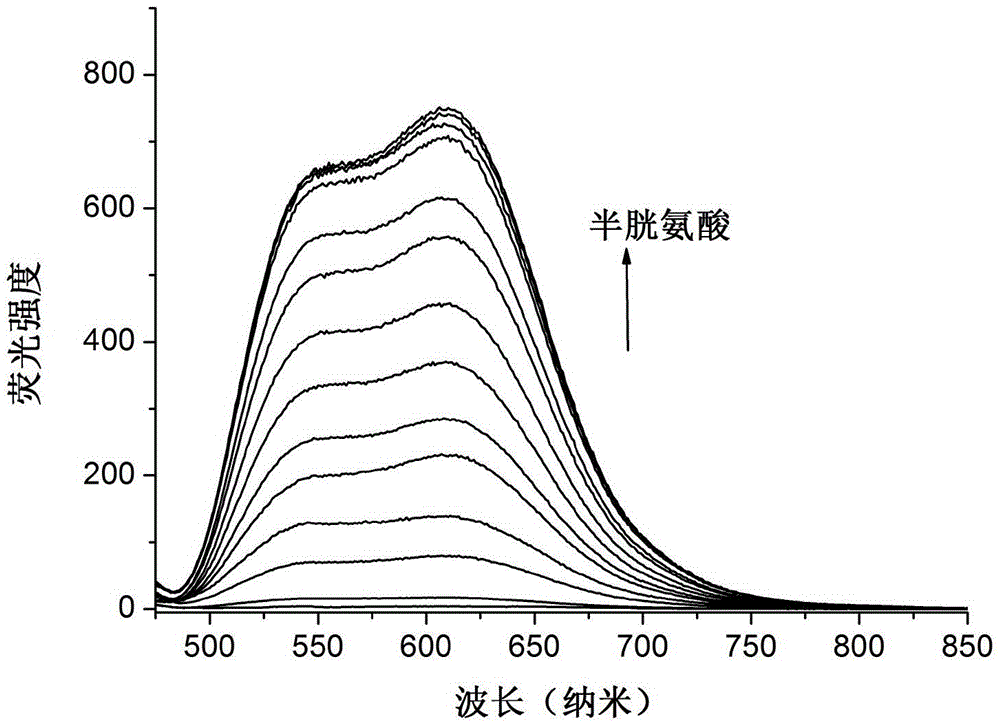
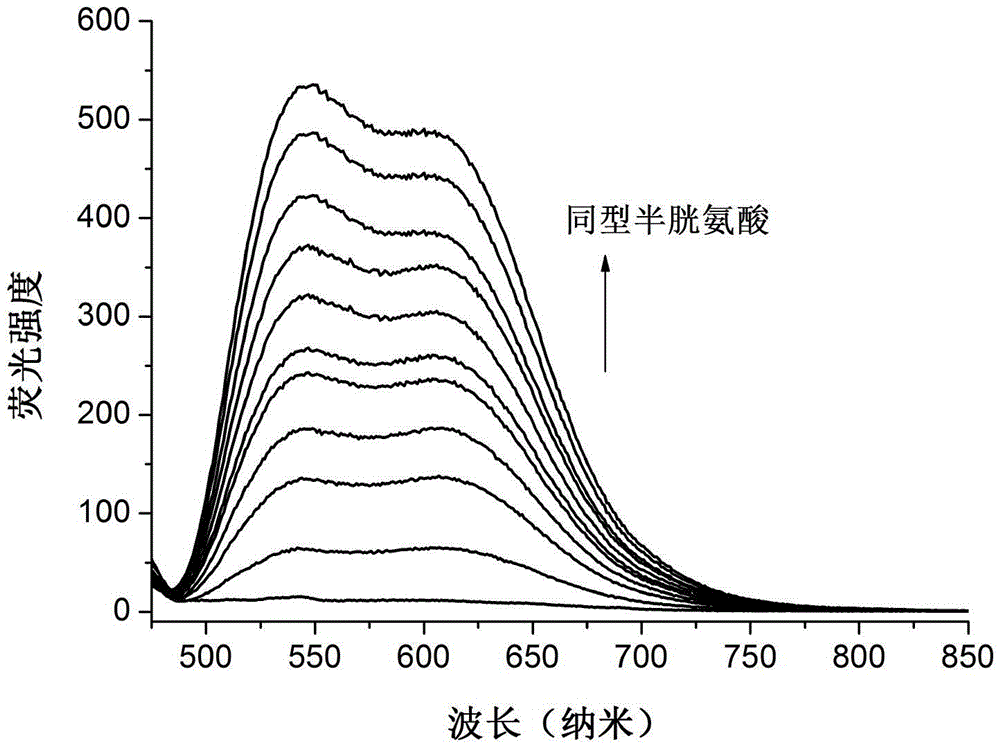
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of compound 1
[0028] 4-(Dimethylamino)cinnamaldehyde (0.3505g, 2.0mmol), potassium hydroxide (0.2076g, 3.7mmol) and o-hydroxyacetophenone (0.2723g, 2.0mmol) were dissolved in 5 mL of anhydrous methanol, Under the protection of argon, it was heated to reflux for 4h. The reaction was stopped, cooled to room temperature, and the reaction system was poured into 200 g of crushed ice to obtain a brownish-yellow clear solution. Use 4M dilute hydrochloric acid to adjust the pH to about 2-3. A large amount of dark solids precipitate out. Suction filtration, followed by deionized water, 10% sodium bicarbonate solution, and vacuum drying. The crude product was recrystallized from absolute ethanol to obtain 0.3988 g of dark purple needle-like crystals, yield: 68%. The structural characterization of the probe molecule is as follows: 1HNMR (400MHz, CDCl3) δ7.82 (dd, J = 8.0, 1.2Hz, 1H), 7.73 (dd, J = 14.4, 11.2Hz, 1H), 7.47–7.38 (m, 3H), 7.12(d, J=14....
Embodiment 2
[0029] Embodiment 2: the synthesis of compound 2
[0030] Dissolve the product 1 (0.1001 g, 0.3 mmol) obtained in the previous step in 20 mL of methanol and 3 mL of 0.5 M sodium hydroxide solution, stir well to dissolve all the solids, and then add 0.33 mL of 30% hydrogen peroxide solution to it. Raise the temperature to 70°C, react for 3 hours, stop heating, cool to room temperature, filter with suction, wash the crude product twice with cold anhydrous methanol, and dry in vacuo to obtain 0.0544 g of red solid powder, yield: 59%. The structural characterization of the probe molecule is as follows: 1HNMR (400MHz, d6-DMSO) δ7.96 (d, J = 8.3Hz, 1H), 7.42 (dd, J = 17.1, 9.0Hz, 3H), 7.33 (d, J = 8.7Hz, 2H), 7.18(dd, J=7.8, 5.1, 2.8Hz, 1H), 6.98(d, J=16.2Hz, 1H), 6.67(d, J=8.8Hz, 2H), 2.87(s, 6H). HRMS (EI) m / z calculated the molecular weight of [C19H17NO3+H]+: 308.1208, found: 308.1278.
Embodiment 3
[0031] Embodiment 3: the synthesis of probe molecule 3
[0032] Compound 2 (0.0451 g, 0.15 mmol) and 4-chloro-7-nitrobenzo-2-oxa-1,3-oxadiazole (NBD-Cl) (0.0307 g, 0.15 mmol) were dissolved in 2 mL of anhydrous Acetonitrile, triethylamine (0.025mL, 0.16mmol) was added, and stirred overnight at room temperature. Stop the reaction, filter with suction, wash twice with absolute ethanol, and dry in vacuo to obtain 0.0371 g of dark brown solid, yield: 56%. The structural characterization of the probe molecule is as follows: 1HNMR (500MHz, d6-DMSO) δ8.61 (d, J = 8.4Hz, 1H), 8.06 (dd, J = 7.9, 1.4Hz, 1H), 7.93–7.81 (m, 3H), 7.60(d, J=8.9Hz, 2H), 7.54(t, J=7.4Hz, 1H), 7.16(d, J=8.4Hz, 1H), 7.00(d, J=15.8Hz, 1H) ,6.71(d,J=9.0Hz,2H),2.99(s,6H).13CNMR(125MHz,d6-DMSO)δ170.06,157.52,155.24,152.67,152.35,145.44,144.83,140.88,138.21,135.81,134.89, 132.66, 130.97, 130.89, 125.77, 125.55, 124.09, 122.53, 118.76, 112.25, 111.44, 109.85, 107.75, 9.27.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com