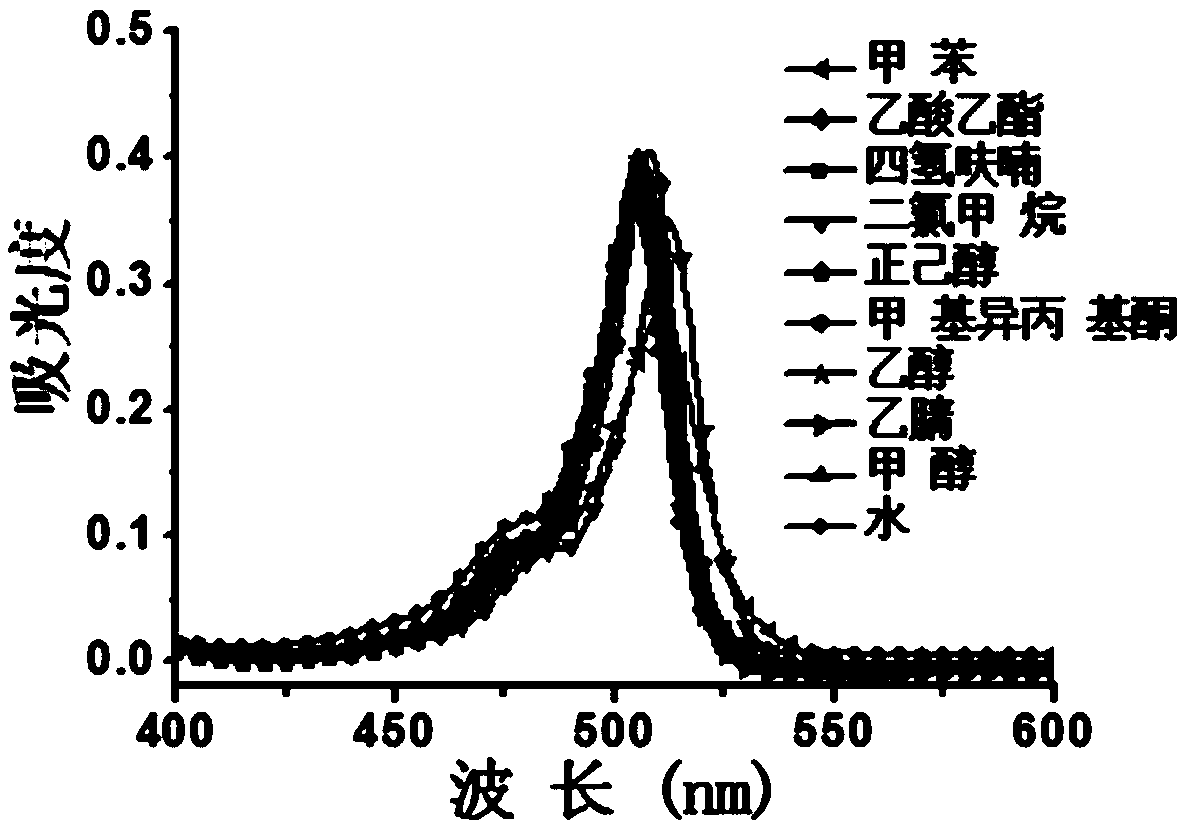
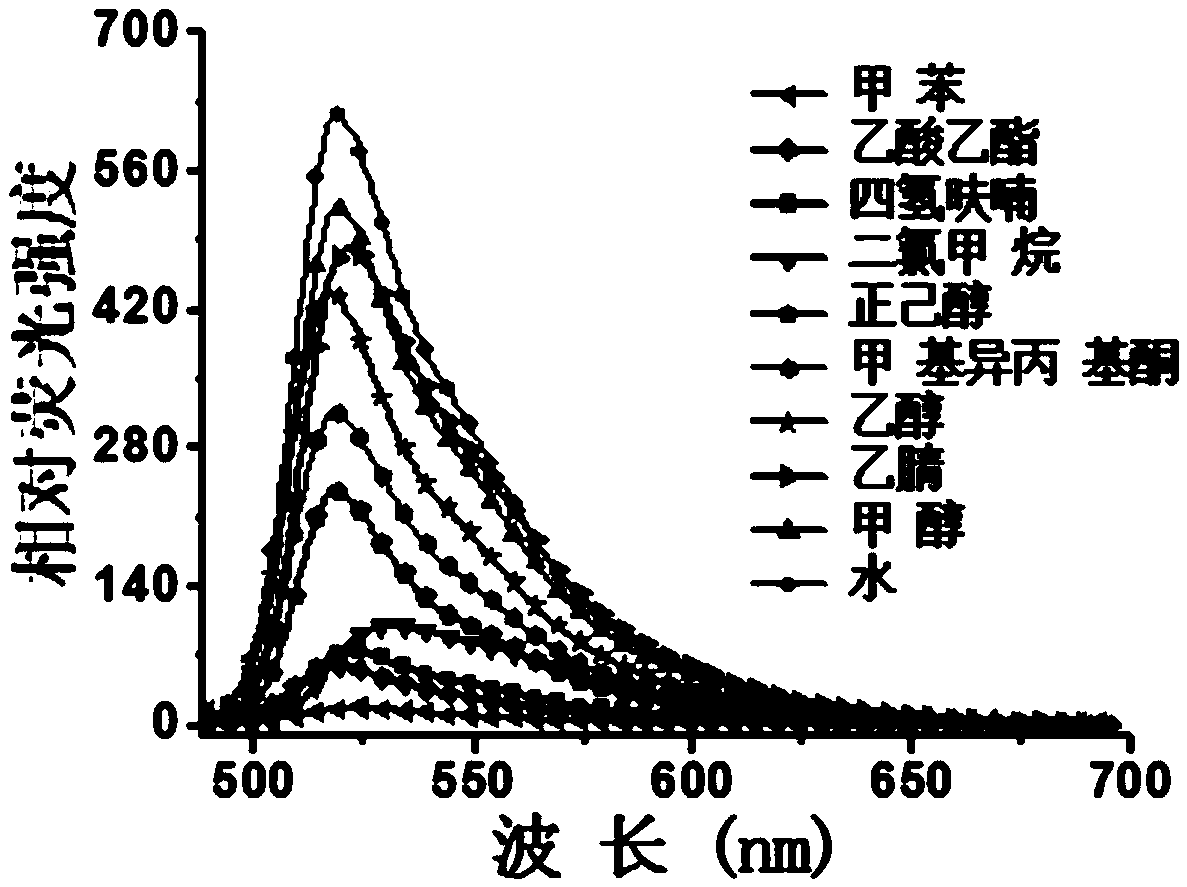
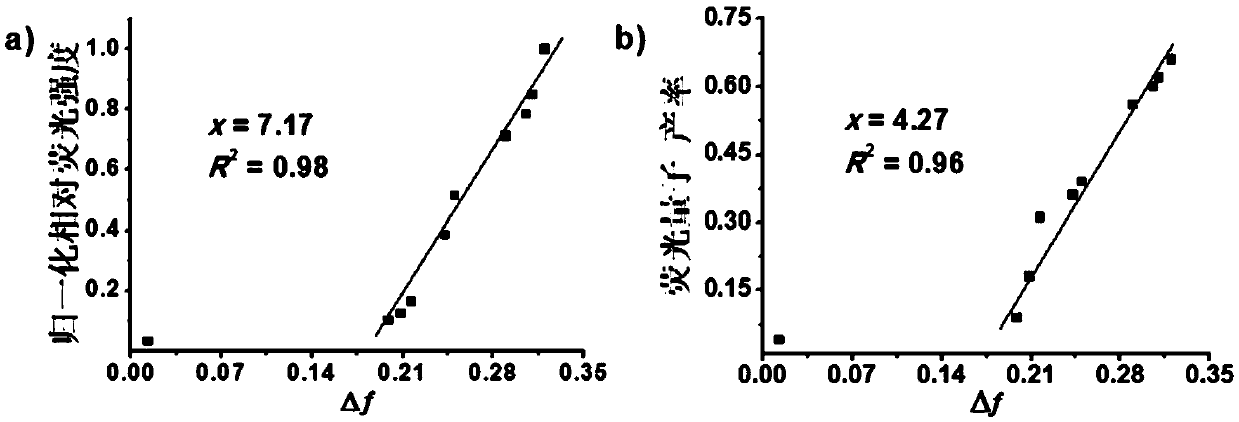
Boron-dipyrrolemethene fluorescent dye and preparation method and application thereof
A dipyrromethene and fluorescent dye technology, which is applied in the direction of methine/polymethine dyes, luminescent materials, organic dyes, etc., to achieve the effects of good biocompatibility, simple synthesis, and easy-to-obtain products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0060] Another specific embodiment, said R 2 is H, methyl or ethyl. R 2 H is most preferred.
[0061] Another specific embodiment, said R 4 and R 5 each independently selected from C 1-4 of alkyl. More preferably, the R 4 and R 5 Each is independently preferably a methyl group or an ethyl group.
[0062] In a further preferred embodiment, said X is iodine.
[0063] In another preferred mode of practice, said n is an integer selected from 1-3, especially preferably n is 2 or 3.
[0064] More specifically, the boron fluoride complexed dipyrromethene fluorescent dye of the present invention is selected from compounds with the following structures:
[0065]
[0066] The present invention further provides a method for preparing boron fluoride complexed dipyrromethene (BODIPY) fluorescent dyes in which n is 1 or 4-8, comprising the following steps: reacting halogenated acid chlorides with pyrrole derivatives to obtain BODIPY dye intermediates, Substitution of secondary...
Embodiment 1
[0096] Synthesis of Fluorescent Dye Compound BP-1
[0097]
[0098] ①Synthesis of intermediate 1
[0099] 2,4-Dimethylpyrrole (2.0 mL, 19 mmol) and chloroacetyl chloride (0.7 mL, 8.8 mmol) were dissolved in 20 mL of dehydrated dichloromethane. The reaction solution was stirred at 0 °C for 30 min, and then at 25 °C for 30 min. Under ice-cooling, triethylamine (3.7 mL, 26 mmol) was added dropwise, and stirred at 25°C for 10 min. Boron trifluoride diethyl ether (5.5 mL, 44 mmol) was added dropwise and stirred overnight at 25°C. The solvent was distilled off under reduced pressure, and intermediate 1 (656 mg, 25.2%) was obtained by column chromatography. 1 HNMR (400MHz, CDCl 3 ), δ: 6.09(s, 2H, pyrrole-H), 4.77(s, 2H, CH 2 ),2.53(s,12H,CH 3 ).
[0100] ②Synthesis of Intermediate 2
[0101] Compound 1 (90.0 mg, 0.3 mmol), dimethylamine (0.15 mL, 2M in THF, 0.3 mmol), potassium iodide (7.5 mg, 0.045 mmol) and potassium carbonate (63.0 mg, 0.45 mmol) were dissolved in 10 m...
Embodiment 2
[0105] Synthesis of fluorescent dye compound BP-2:
[0106]
[0107] ①Synthesis of Intermediate 4
[0108] Compound 3 (82.0 mg, 0.314 mmol) was dissolved in 3 mL of dehydrated dichloromethane, and dimethylamine (3.14 mL, 2M in THF, 6.28 mmol) was added. The reaction solution was refluxed and stirred for three days under the protection of nitrogen, diluted with dichloromethane, and then washed with saturated NH 4 The crude product was obtained by washing with aqueous Cl solution and brine. Purified by column chromatography to obtain intermediate 4 (60.0 mg, 60.1%). 1 HNMR (400MHz, CDCl 3 ), δ: 6.06(s, 2H, pyrrole-H), 3.20(t, J=8Hz, 2H, CH 2 ), 2.56(t, J=8Hz, 2H, CH 2 ),2.51(s,6H,CH 3 ),2.47(s,6H,CH 3 ),2.35(s,6H,CH 3 ); 13 CNMR (100MHz, CDCl 3 ), δ: 154.2, 140.4, 137.4, 131.7, 121.8, 60.6, 45.4, 27.1, 16.6, 14.5; TOF (C 17 h 25 BF 2 N 3 + ) Theoretical value: 320.2104, experimental value: 320.2115.
[0109] ②Synthesis of Compound BP-2
[0110] Intermediate 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com