Pharmaceutical composition for treating radiation proctitis
A composition and drug technology, applied in directions such as drug combination, drug delivery, and pharmaceutical formula, can solve the problems of not disclosing the dosage and ratio of various ingredients, not providing formula experimental data and effect verification, etc., and achieving a short drug action time. , Improve the local cell microenvironment and limit the effect of damage expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
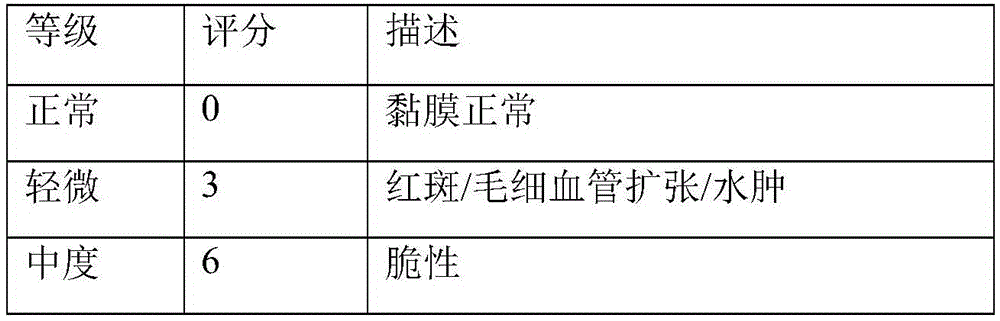
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of drug compound
[0027] The raw materials used in this example are: 10 g of sucralfate, 0.1 g of lidocaine, 5 mg of dexamethasone, 80,000 units of gentamicin, 10,000 units of recombinant epidermal growth factor and 100 ml of normal saline.
[0028] Sucralfate can use the existing commercial product "Sucralfate Suspension", the specification is 10ml / cartridge, the effective content is 1g / cartridge, and a total of 10 sticks are used.
[0029] The lidocaine can adopt the existing commercial product "lidocaine hydrochloride", the specification is 5ml each, the effective dose is 1g, and a total of one is used.
[0030] Dexamethasone can use the existing commercial product "Dexamethasone Sodium Phosphate Injection", the specification is 1ml each, containing dexamethasone 5mg, a total of 1 bottle is used.
[0031] Gentamicin can use the existing commercial product "Gentamycin Injection", the specification is 2ml per vial, containing 80,000 unit...
Embodiment 2
[0034] Embodiment 2: the use method of drug compound
[0035] The above-mentioned drug complex is placed in an enema bag, and the temperature of the liquid is controlled at about 40 degrees Celsius. The patient lies on the left side, the anus of the enema bag is short and gently inserted into the anal canal, the insertion depth is 20-25cm, the infusion switch is turned on, and the flow rate is controlled at 60-80 drops per minute. After the infusion, the patient kept the left side lying position for 30 minutes and then returned to the supine position, keeping the enema solution for as long as possible.
Embodiment 3
[0036] Embodiment 3: the clinical application effect of drug compound
[0037] 1. Subject selection
[0038] Diagnostic criteria: Select patients with a history of radiotherapy for abdominal and pelvic tumors in the past, and patients with late rectal reactions such as difficulty in defecation, thin or frequent defecation, loose stools, blood in the stool, and falling pain during defecation. According to the National Occupational Health Standard of the People's Republic of China "Diagnostic Criteria for Radiation Proctitis" GBZ111-2002, it was diagnosed as radiation proctitis.
[0039] Inclusion criteria: a history of radiation therapy for pelvic tumors; age: 18-75 years old; male or female; hospitalized patients; agree to participate in this trial and sign an informed consent form
[0040] Exclusion criteria: Pregnant women; patients with acute abdomen; patients with anus, rectum, and contact surgery; patients with fecal incontinence; brain diseases, abnormal judgment abilit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com