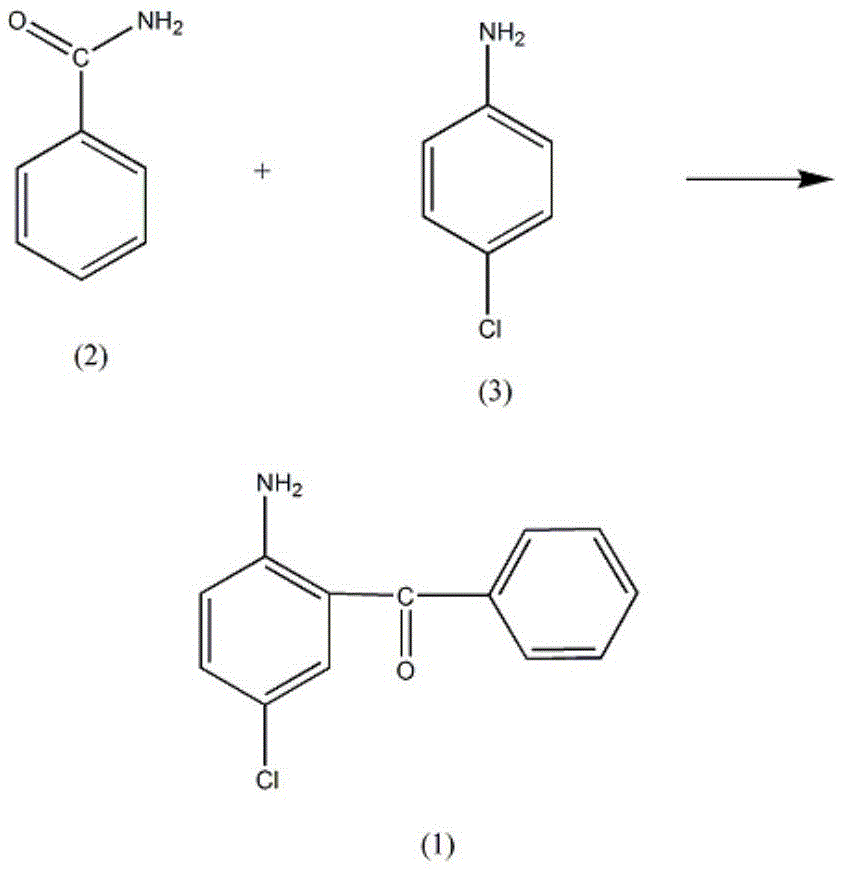
Synthetic method of Oxazolam drug intermediate 2-amino-5-chlorobenzophenone
A technology of oxazolam and chlorobenzophenone, which is applied in the field of synthesis of 2-amino-5-chlorobenzophenone, an intermediate of oxazolam medicine, can solve the problem of inability to recall, loss and transient anterograde in patients Memory and other problems, to achieve the effect of reducing reaction temperature and reaction time, reducing intermediate links, and improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0013] In a reaction vessel equipped with a stirrer, a thermometer and a reflux condenser, add 3.51 mol of benzamide (2), raise the temperature of the solution to 120°C, add 1.41 mol of p-chloroaniline (3) dropwise, and slowly heat up after the addition To 190°C, add 5.5mol of stannous chloride, increase the solution temperature to 240-250°C, react at this temperature for 3h, until no gas is generated, lower the solution temperature to 90°C, add 10% chloride by mass Potassium solution 500ml, raise the solution temperature to 130°C and reflux, after 3h, pour out the water layer, after cooling the solution, a dark suspension is precipitated, this suspension is added to 2.5L of 50% phosphoric acid solution by mass fraction, reflux for 20h, After cooling, pour the solution into 1200ml of 20% sodium nitrate solution by mass fraction, reduce the temperature of the solution to 5°C, add 80% cyclohexane for extraction, and add 300ml of 30% sodium sulfite solution to the cyclohexane laye...
example 2
[0015] In a reaction vessel equipped with a stirrer, a thermometer, and a reflux condenser, add 3.53 mol of benzamide (2), raise the temperature of the solution to 121°C, add 1.41 mol of p-chloroaniline (3) dropwise, and slowly raise the temperature after the addition To 192°C, add 5.7 mol of stannous chloride, increase the solution temperature to 246°C, react at this temperature for 3 hours, until no gas is generated, lower the solution temperature to 92°C, and add a 12% potassium chloride solution 500ml, raise the temperature of the solution to reflux at 130°C, after 3h, pour out the water layer, after cooling the solution, a dark suspended substance precipitates, add the suspended substance to 2.5L of 52% phosphoric acid solution, reflux for 21h, after cooling , Pour the solution into 1200ml mass fraction of 22% sodium nitrate solution, reduce the solution temperature to 6°C, add mass fraction of 82% cyclohexane for extraction, add mass fraction of 32% sodium sulfite solutio...
example 3
[0017] In a reaction vessel equipped with a stirrer, a thermometer, and a reflux condenser, add 3.55 mol of benzamide (2), raise the temperature of the solution to 123°C, add 1.41 mol of p-chloroaniline (3) dropwise, and slowly heat up after the addition To 195°C, add 5.9 mol of stannous chloride, raise the solution temperature to 250°C, react at this temperature for 4h, until no gas is generated, lower the solution temperature to 95°C, add a mass fraction of 15% potassium chloride solution 500ml, raise the temperature of the solution to reflux at 130°C, after 4 hours, pour out the water layer, after cooling the solution, a dark suspended substance precipitates, add the suspended substance to 2.5L of 55% phosphoric acid solution, reflux for 23h, after cooling , Pour the solution into 1200ml mass fraction of 25% sodium nitrate solution, reduce the temperature of the solution to 8°C, add mass fraction of 85% cyclohexane for extraction, add mass fraction of 35% sodium sulfite solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com