Octakis (acetylenyl dimethyl siloxane) polysilsesquioxane and synthetic method thereof
A technology of ethynyl dimethyl siloxane and ethynyl dimethyl chlorosilane, which is applied in the field of cage octapolysilsesquioxane and its synthesis, can solve the problem of less terminal alkynyl POSS and achieve high reactivity , good development and application prospects, and the effect of excellent high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
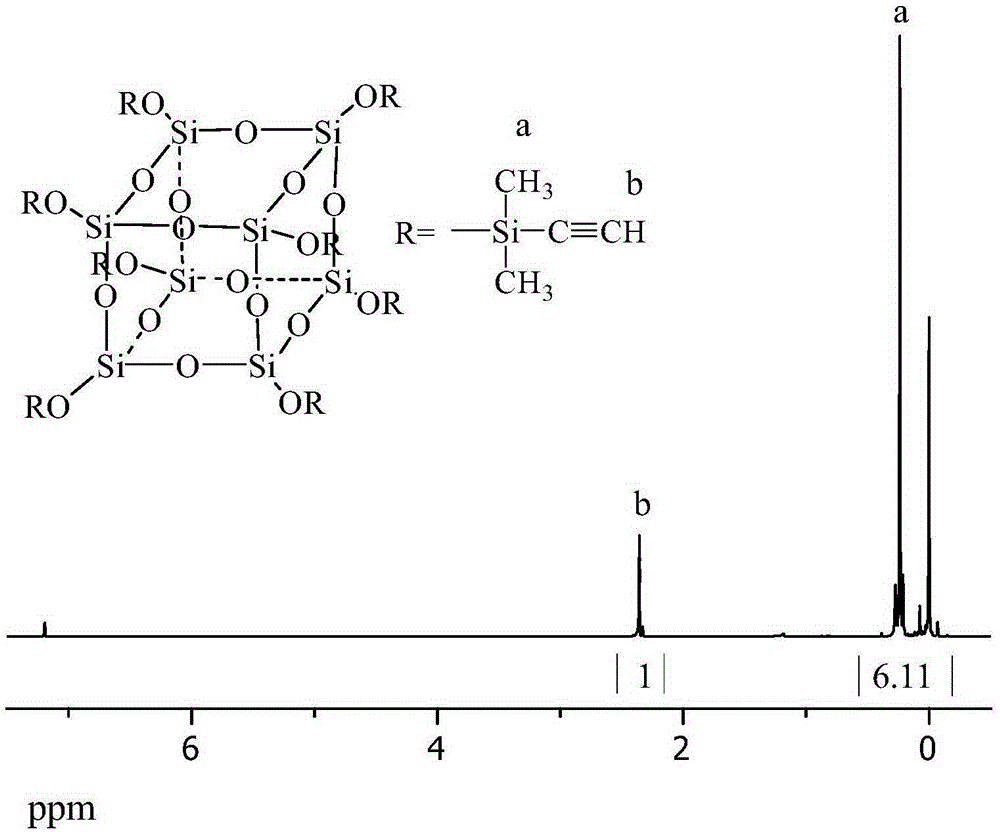
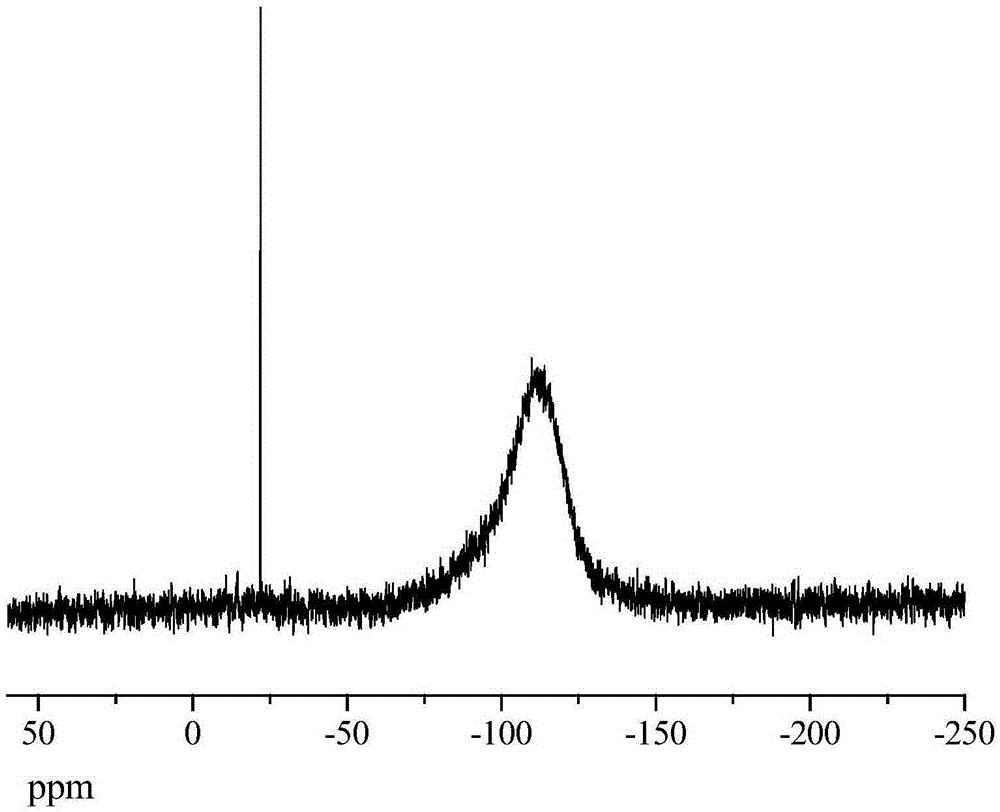
[0058] Synthesis of Cage Octa(ethynyldimethylsiloxy)silsesquioxane
[0059] 1). Synthesis of 1,3-diethynyl-1,1,3,3-tetramethyldisiloxane
[0060] 20ml of 1,3-dichloro-1,1,3,3-tetramethyldisiloxane, 100ml of acetone, 400ml of ethynyl magnesium chloride of 0.5MTHF and Cu(PPh 3 ) 3 Add 0.11 g of Br into a 500 ml four-neck round-bottomed flask equipped with a condenser, protect it with nitrogen, stir it magnetically, heat the reactant to 55° C., and react at a constant temperature for 8 hours. The solution was concentrated to about 50ml, and a large amount of precipitate was formed, which was washed with n-pentane and filtered. The filtrate was distilled under reduced pressure to obtain 1,3-diethynyl-1,1,3,3-tetramethyldisiloxane as a colorless transparent liquid with a yield of 46% and a purity of 97.7%. 1 HNMR (400MHz, CDCl 3 ): δ0.3(m, 6H, -CH 3 ), 2.4 (m, H, ≡CH).
[0061] 2).Synthesis of ethynyldimethylchlorosilane
[0062] Add 3.01 g of 1,3-diethynyl-1,1,3,3-tetrameth...
Embodiment 2
[0068] Synthesis of Cage Octa(ethynyldimethylsiloxy)silsesquioxane
[0069] 1). Synthesis of 1,3-diethynyl-1,1,3,3-tetramethyldisiloxane
[0070] 20ml of 1,3-dichloro-1,1,3,3-tetramethyldisiloxane, 100ml of toluene, 400ml of ethynylmagnesium chloride of 0.5MTHF and Cu(PPh 3 ) 3 Add 0.22 g of Br into a 500 ml four-necked round-bottomed flask equipped with a condenser, protect it with nitrogen gas, stir it magnetically, heat the reactant to 80° C., and react at a constant temperature for 1 h. The solution was concentrated to about 50ml, and a large amount of precipitate was formed, which was washed with n-pentane and filtered. The filtrate was distilled under reduced pressure to obtain 1,3-diethynyl-1,1,3,3-tetramethyldisiloxane as a colorless and transparent liquid product with a yield of 48% and a purity of 97.4%. 1 HNMR (400MHz, CDCl 3 ): δ0.3(m, 6H, -CH 3 ), 2.4 (m, H, ≡CH).
[0071] 2).Synthesis of ethynyldimethylchlorosilane
[0072] Add 3.01 g of 1,3-diethynyl-1,1,...
Embodiment 3
[0076] Synthesis of Cage Octa(ethynyldimethylsiloxy)silsesquioxane
[0077] 1). Synthesis of 1,3-diethynyl-1,1,3,3-tetramethyldisiloxane
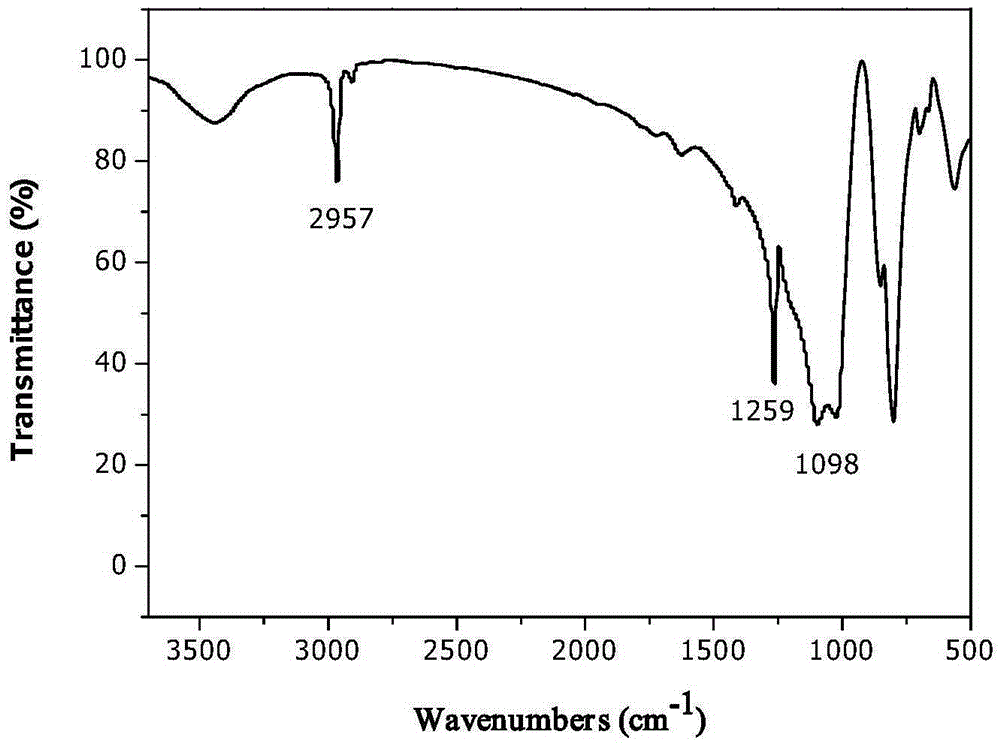
[0078] 20ml of 1,3-dichloro-1,1,3,3-tetramethyldisiloxane, 100ml of DMF, 400ml of ethynyl magnesium chloride of 0.5MTHF and Cu(PPh 3 ) 3 Add 0.33 g of Br into a 500 ml four-necked round-bottomed flask equipped with a condenser, protect it with nitrogen gas, stir it magnetically, heat the reactant to 100° C., and react at a constant temperature for 1 h. The solution was concentrated to about 50ml, and a large amount of precipitate was formed, which was washed with n-pentane and filtered. The filtrate was distilled under reduced pressure to obtain 1,3-diethynyl-1,1,3,3-tetramethyldisiloxane as a colorless transparent liquid with a yield of 56% and a purity of 97.6%. FTIR (KBr, cm -1 ): 1098 (Si-O-Si), 1259 (Si-C), 2957 (≡CH); 1 HNMR (400MHz, CDCl 3 ): δ0.3(m, 6H, -CH 3 ), 2.4 (m, H, ≡CH).
[0079] 2).Synthesis of ethynyldimethylchloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com