Compositions and methods for treatment of type 1 diabetes
A composition, technology of BHT-3021, applied in botany equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
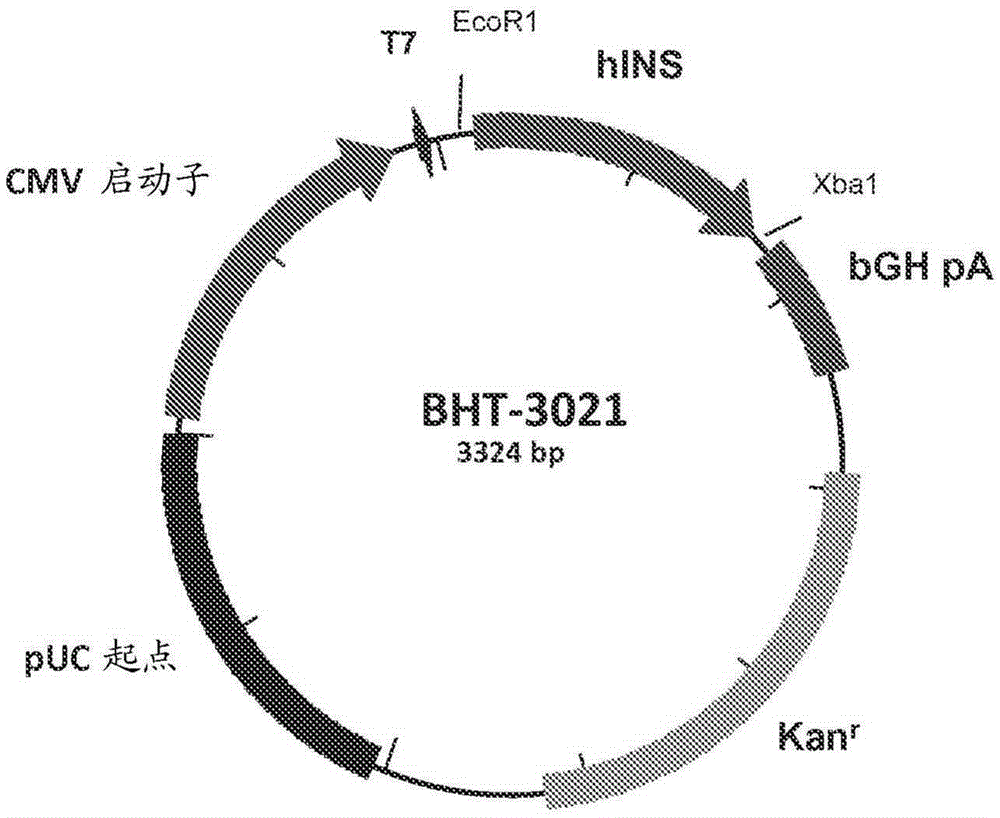
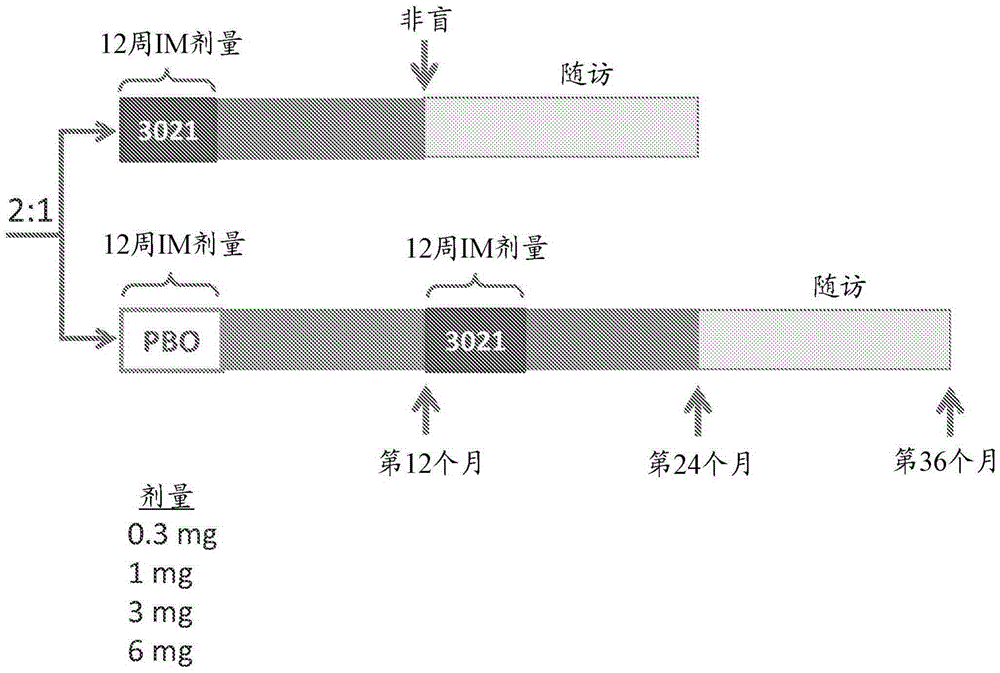
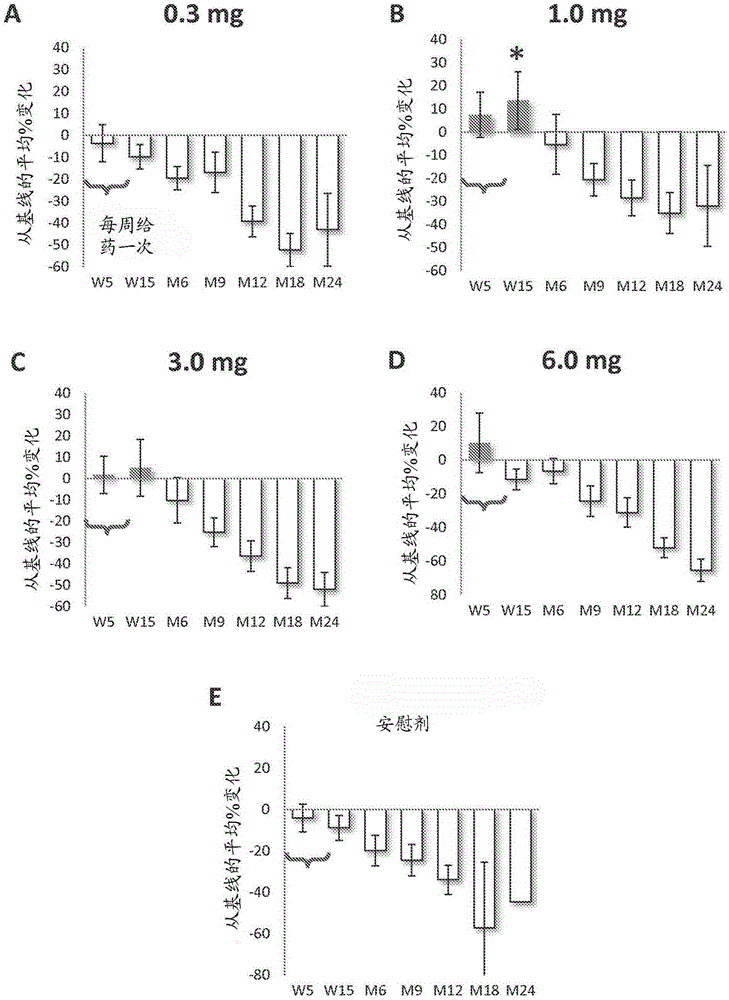
[0073] In type 1 diabetes, there is a strong inflammatory response that destroys islets in the pancreas, the site of insulin production and release. In type 1 diabetes (T1D), proinsulin is a major target of the adaptive immune response. The present invention provides an engineered DNA plasmid (BHT-3021) encoding proinsulin that preserves beta cell function in T1D patients by reducing insulin-specific CD8 T cells. We studied 80 subjects over the age of 18 who had been diagnosed with TlD within the past five years. Subjects were randomized 2:1 to receive intramuscular injections of BHT3021 or BHT-placebo once weekly for 12 weeks, followed by blinded monitoring of safety and immune responses. Four dose levels of BHT-3021 were evaluated: 0.3 mg, 1.0 mg, 3.0 mg, and 6.0 mg. C-peptide was used as an efficacy measure and a safety measure for the investigation. Islet-specific CD8 T cell frequencies were assessed using multimers of HLA class I monomers loaded with pancreas-derived p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com