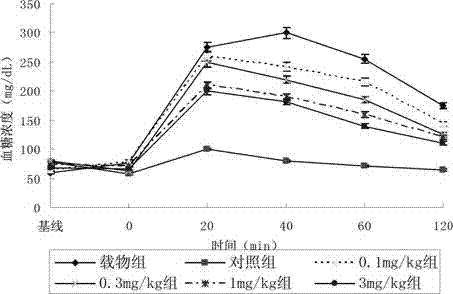
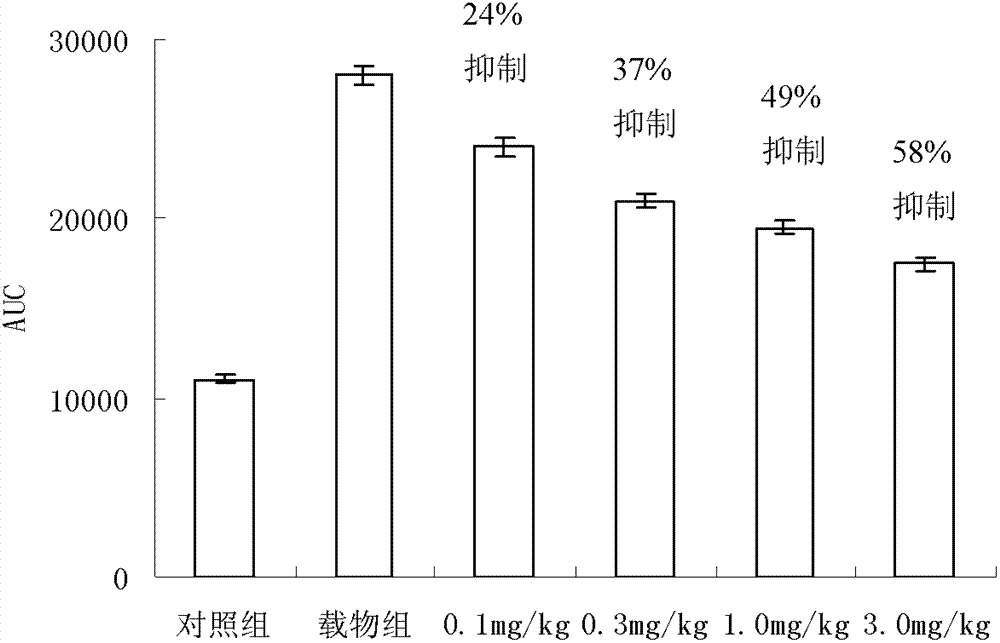
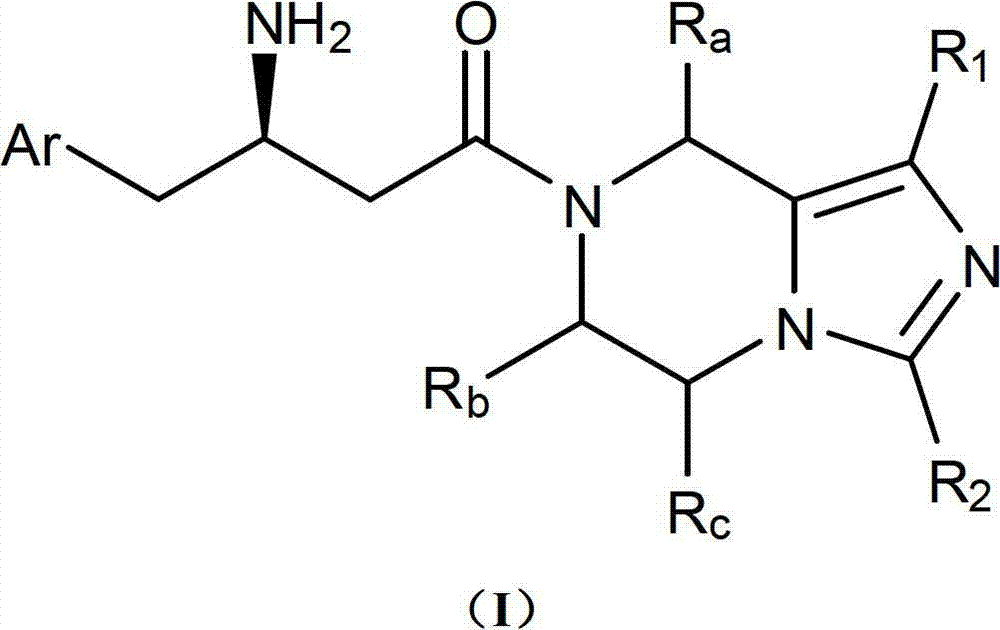
Compound serving as dipeptidyl enzyme inhibitor and composition thereof and applications of compound and composition
A compound, alkyl technology, applied in the field of disease compounds, type II diabetes, prevention or treatment of diseases related to dipeptidyl peptidase-IV enzyme, can solve the problem that DPP-IV inhibitors are not widely studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0167] Synthetic Route 1 of Diastereomers 1 and 2
[0168]
[0169] Step 1: Synthesis of 3-methylpyrazine-2-carbonitrile (1A)
[0170] Dissolve 173 grams (1 mole) of 2-bromo-3-methylpyrazine in 2 liters of DMF, then add 121.2 grams (1.2 moles) of triethylamine, 97.5 grams (1.2 moles) of zinc cyanide, and tetrakistriphenyl 17.3 grams of phosphopalladium (catalytic amount), the resulting solution was heated to 100°C under nitrogen protection, reacted for 24 hours, cooled, poured into 2 liters of ice water, extracted three times with 500 milliliters of ethyl acetate, combined the organic phases, washed with water, Washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 159 g of product with a yield of 75%.
[0171] Step 2: Synthesis of (3-methylpyrazin-2-yl)methylammonia (1B)
[0172] 59.56 grams (0.5 moles) of compound 1A were dissolved in 1 liter of methanol, 6 grams of 10% palladium carbon was added, and the resulting solu...
Embodiment 3 and 4
[0185] Diastereomers 3 and 4
[0186] Synthesis path 2
[0187]
[0188] Step 1: Synthesis of tert-butyl 8-methyl-3-trifluoromethyl-5,6-dihydroimidazo[1,5-a]pyrazine-7(8H)-carboxylate (3A)
[0189] Dissolve 20.5 g (0.1 mol) of compound 1E in 200 ml of dichloromethane, add 12.12 g (0.12 mol) of triethylamine, and add (Boc) under ice water cooling 2 O25.96 g (0.12 mol), reacted at room temperature for 2 hours, poured into 1 liter of ice water, washed the organic phase with water, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 27.5 g of the product, with a yield of 90%.
[0190] Step 2: Synthesis of tert-butyl 1-bromo-8-methyl-3-trifluoromethyl-5,6-dihydroimidazo[1,5-a]pyrazine-7(8H)-carboxylate (3B)
[0191] 15.3 g (0.05 mol) of compound 3A was dissolved in 200 ml of dichloromethane, 8.9 g (0.05 mol) of NBS was added, reacted at room temperature for 24 hours, poured into 500 ml of ice water, and the organic phas...
Embodiment 5 and 6
[0202] Diastereomers 5 and 6
[0203] Synthesis path 3
[0204]
[0205] Step 1: Synthesis of 7-[(R)-3-(tert-butoxycarboxamido)-4-(2,4,5-trifluorophenyl)butyryl)]-8-methyl-3-tri Fluoromethyl-5,6,7,8-tetrahydroimidazo[1,5-a]pyrazine-1-carboxylic acid (5A)
[0206] 2.89 g (0.005 mol) of compound 3E was dissolved in 20 ml of methanol and 10 ml of water, added 0.24 g (0.01 mol) of lithium hydroxide, reacted at room temperature for 24 hours, added 1N hydrochloric acid to adjust the pH to 7, extracted three times with 20 ml of ethyl acetate , the organic phases were combined, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain a crude product.
[0207] Step 2: Synthesis of 7-[(R)-3-amino-4-(2,4,5-trifluorophenyl)butanoyl)]-8-methyl-3-trifluoromethyl-5,6, 7,8-tetrahydroimidazo[1,5-a]pyrazine-1-carboxylic acid (5 and 6)
[0208] According to the method of Step 8 in Examples 1 and 2, 5A was deprotected to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com