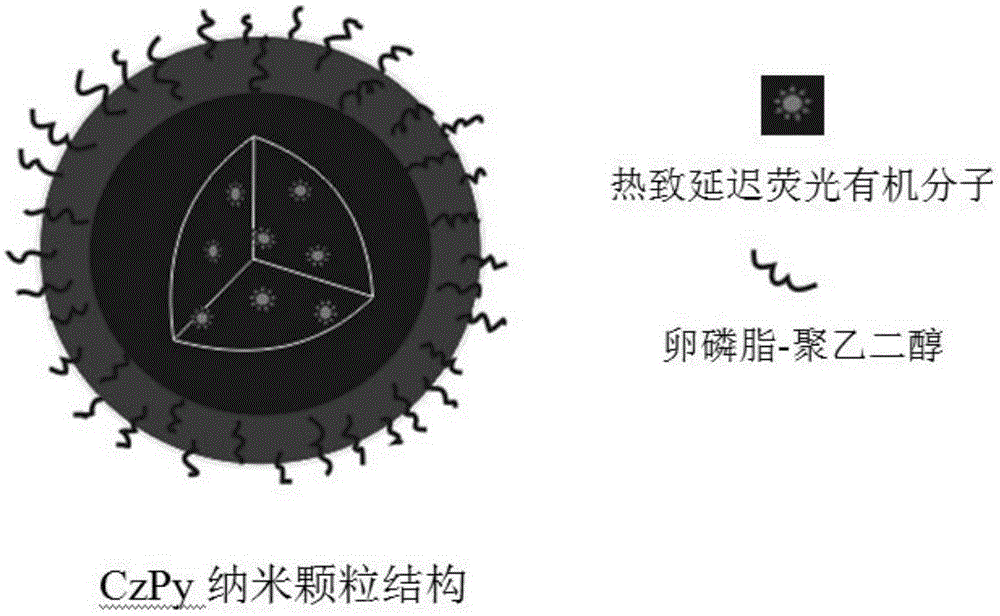
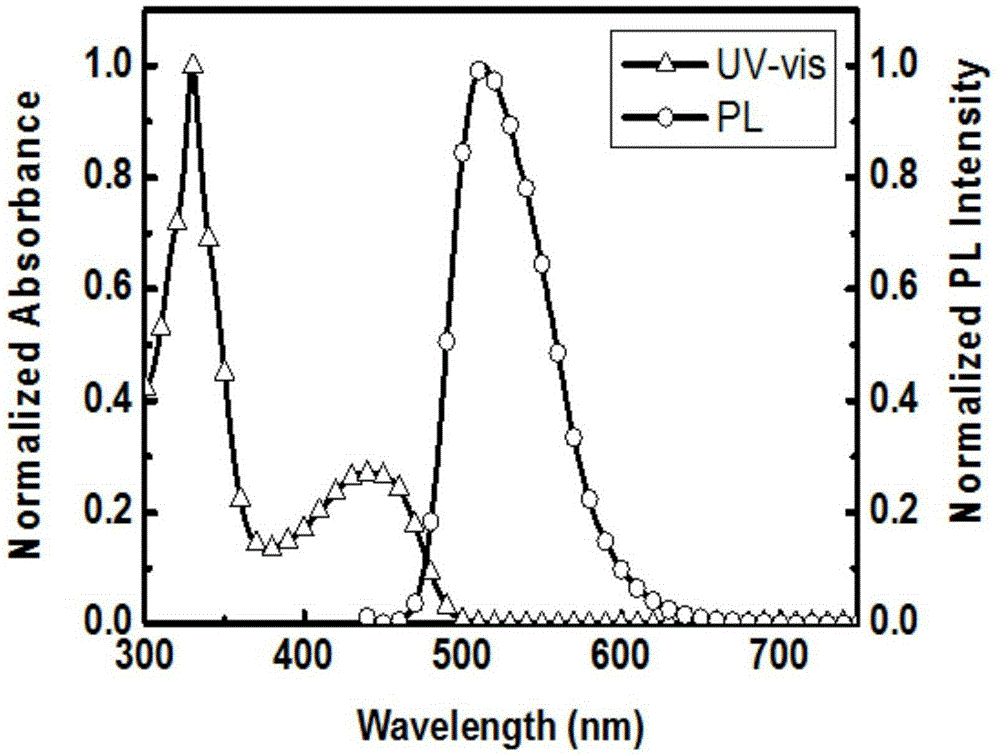
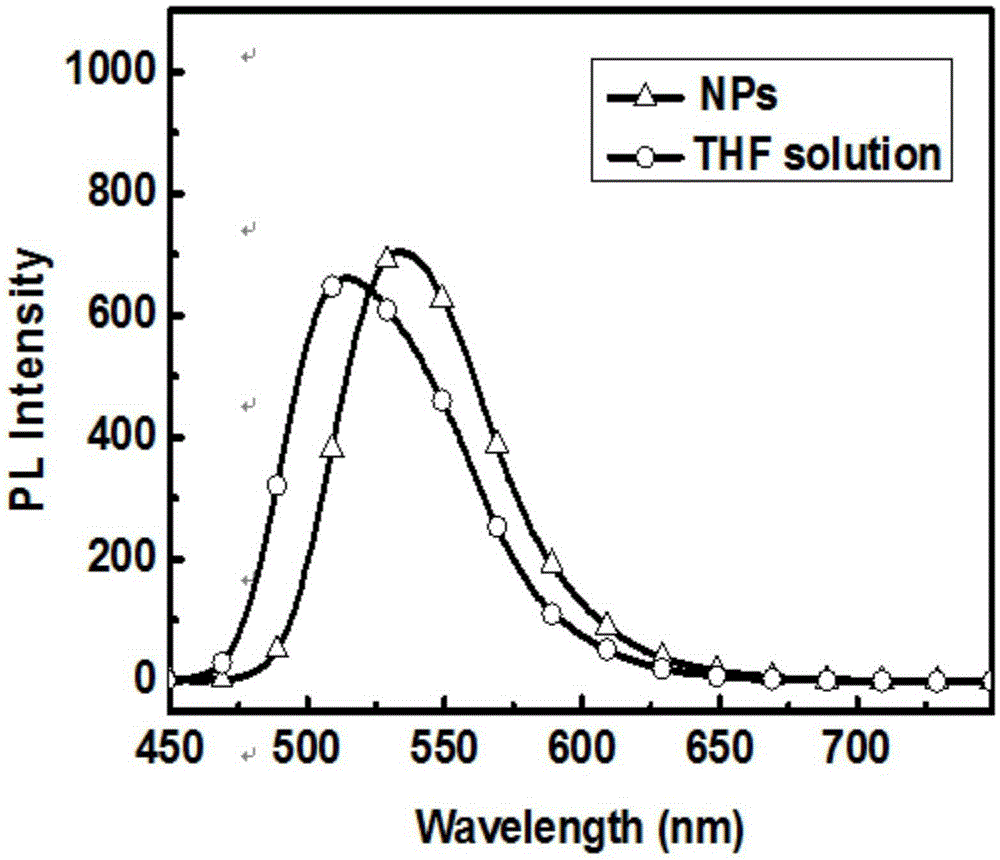
Preparation method and time-resolved biological imaging application of thermally activated delayed long-life fluorescent organic material-based nanoparticles
A technology of thermally induced delayed fluorescence and fluorescent nanoparticles, which is applied in the fields of luminescent materials, material excitation analysis, organic chemistry, etc., can solve the problems that the signal is easily affected by the environment, the sensitivity and signal-to-noise ratio are low, and the hydrophobicity is easy to aggregate. Good fluorescence lifetime stability, long fluorescence lifetime, uniform size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a two-neck flask (100mL), dissolve 2-bromonitrobenzene (2.01g, 10mmol) and 2-thiopheneboronic acid (1.42g, 11mmol) in 30mL of toluene, inject 20mL of 2M potassium carbonate solution, and pump three times. Under nitrogen protection, tetrakis(triphenylphosphine)palladium (150mg, 0.14mmol) was added, heated to 90°C, and reacted for 16 hours. Naturally cooled to room temperature, extracted with dichloromethane, dried, concentrated, and separated by column chromatography to obtain 1.85 g of bright yellow powder with a yield of 81%. 1 HNMR (400MHz, CDCl3 )δ7.95(d, J=1.9Hz, 1H), 7.77(dd, J=8.1, 1.9Hz, 1H), 7.56(d, J=8.1Hz, 1H), 7.42(d, J=1.0Hz, 1H), 7.39(dd, J=5.1, 1.1Hz, 1H), 7.11(d, J=1.4Hz, 1H). 13 CNMR (101MHz, CDCl 3 ) δ 141.03, 132.68, 128.69, 128.53, 127.94, 127.50, 127.24, 127.21, 126.71, 124.88, 120.74.
[0035]
[0036] Add 2-nitro-phenylthiophene (9.37g, 33mmol), triphenylphosphine (22.7g, 100mmol) and chlorobenzene (60mL) into a two-necked round bottom fla...
Embodiment 2
[0043] Add the above-mentioned benzothienopyrrole (6.05g, 48mmol), 2,3,5,6-tetrafluoro-4-cyanopyridine (1.76g, 10mmol), potassium carbonate (9.6g, 70mmol) and dimethyl sulfoxide (100mL), under nitrogen protection, stirred at 150°C for 24h. Extract with dichloromethane and dry over anhydrous sodium sulfate. Concentrate and separate on a silica gel column to obtain CzPy, 6.34g, with a yield of 82%. 1 HNMR (400MHz, CDCl 3 )δ7.59(d, J=8.4Hz, 1H), 7.50(d, J=1.6Hz, 1H), 7.39(d, J=5.2Hz, 1H), 7.27-7.24(dd, J=1.7Hz, 1H), 7.02(d, J=5.2Hz, 1H), 4.06(d, J=7.5Hz, 2H), 2.04(m, 1H), 1.28(m, 24H), 0.84-0.89(m, 6H). 13 CNMR (101MHz, CDCl 3 )δ146.16,142.42,127.29,122.03,121.30,119.97,115.84,115.73,113.02,110.56,49.79,38.37,31.88,31.78,31.68,31.6,29.90,29.58,29.50,29.27,26.4,26.40,22.68,22.63,14.14 , 14.10.
[0044]
[0045]
[0046]
[0047] Example 3
Embodiment 3
[0049] In a two-neck flask (100 mL), 2-bromonitrobenzene (2.01 g, 10 mmol), 2-naphthylboronic acid (1.86 g, 11 mmol) were dissolved in 30 mL of tetrahydrofuran, and 20 mL of 2M potassium carbonate solution was injected. The gas was exchanged three times. Under the protection of nitrogen, tetrakis(triphenylphosphine)palladium (150mg, 0.14mmol) was added, heated to 90°C, and reacted for 16 hours. Naturally cooled to room temperature, extracted with dichloromethane, dried, concentrated, and separated by column chromatography to obtain 1.67 g of bright yellow powder with a yield of 65%. 1 HNMR (400MHz, CDCl 3 )δ8.05(d,J=1.9Hz,1H),8.00(m,3H),7.90(t,2H),7.75(m,1H),7.64(s,1H),7.56(d,J=8.1 Hz, 2H). 13 CNMR (101MHz, CDCl 3 ) δ 141.13, 136.68, 134.21, 133.1, 132.7, 130.45, 128.5, 128.2, 128.1, 127.7, 126.69, 124.4.
[0050]
[0051] Add 2-nitro-phenylbinaphthyl (2.5g, 10mmol), triphenylphosphine (6.3g, 28mmol) and chlorobenzene (22mL) into a two-necked round bottom flask (250mL),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com